- Record: found
- Abstract: found
- Article: found
Hyperglycemia-Induced Reactive Oxygen Species Toxicity to Endothelial Cells Is Dependent on Paracrine Mediators
Read this article at
Abstract
OBJECTIVE—This study determined the effects of high glucose exposure and cytokine treatment on generation of reactive oxygen species (ROS) and activation of inflammatory and apoptotic pathways in human retinal endothelial cells (HRECs).
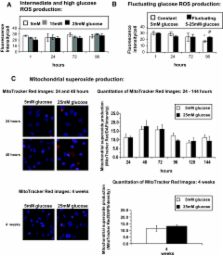
RESEARCH DESIGN AND METHODS—Glucose consumption of HRECs, human retinal pigment epithelial cells (HRPEs), and human Müller cells (HMCs) under elevated glucose conditions was measured and compared with cytokine treatment. Production of ROS in HRECs was examined using 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H 2DCFDA), spin-trap electron paramagnetic resonance, and MitoTracker Red staining after high glucose and cytokine treatment. The activation of different signaling cascades, including the mitogen-activated protein kinase pathways, tyrosine phosphorylation pathways, and apoptosis by high glucose and cytokines in HRECs, was determined.
RESULTS—HRECs, in contrast to HRPEs and HMCs, did not increase glucose consumption in response to increasing glucose concentrations. Exposure of HRECs to 25 mmol/l glucose did not stimulate endogenous ROS production, activation of nuclear factor-κB (NF-κB), extracellular signal–related kinase (ERK), p38 and Jun NH 2-terminal kinase (JNK), tyrosine phosphorylation, interleukin (IL)-1β, or tumor necrosis factor-α (TNF-α) production and only slightly affected apoptotic cell death pathways compared with normal glucose (5 mmol/l). In marked contrast, exposure of HRECs to proinflammatory cytokines IL-1β or TNF-α increased glucose consumption, mitochondrial superoxide production, ERK and JNK phosphorylation, tyrosine phosphorylation, NF-κB activation, and caspase activation.
CONCLUSIONS—Our in vitro results indicate that HRECs respond to cytokines rather than high glucose, suggesting that in vivo diabetes–related endothelial injury in the retina may be due to glucose-induced cytokine release by other retinal cells and not a direct effect of high glucose.
Related collections
Most cited references43
- Record: found
- Abstract: not found
- Article: not found
Diabetes
- Record: found
- Abstract: found
- Article: not found
Diabetic retinopathy: seeing beyond glucose-induced microvascular disease.
- Record: found
- Abstract: found
- Article: not found