- Record: found
- Abstract: found
- Article: found
Who are vaccine champions and what implementation strategies do they use to improve adolescent HPV vaccination? Findings from a national survey of primary care professionals
Read this article at
Abstract
Background
Implementation science researchers often cite clinical champions as critical to overcoming organizational resistance and other barriers to the implementation of evidence-based health services, yet relatively little is known about who champions are or how they effect change. To inform future efforts to identify and engage champions to support HPV vaccination, we sought to describe the key characteristics and strategies of vaccine champions working in adolescent primary care.
Methods
In 2022, we conducted a national survey with a web-based panel of 2527 primary care professionals (PCPs) with a role in adolescent HPV vaccination (57% response rate). Our sample consisted of pediatricians (26%), family medicine physicians (22%), advanced practice providers (24%), and nursing staff (28%). Our survey assessed PCPs’ experience with vaccine champions, defined as health care professionals “known for helping their colleagues improve vaccination rates.”
Results
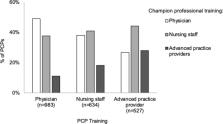
Overall, 85% of PCPs reported currently working with one or more vaccine champions. Among these 2144 PCPs, most identified the champion with whom they worked most closely as being a physician (40%) or nurse (40%). Almost all identified champions worked to improve vaccination rates for vaccines in general (45%) or HPV vaccine specifically (49%). PCPs commonly reported that champion implementation strategies included sharing information (79%), encouragement (62%), and vaccination data (59%) with colleagues, but less than half reported that champions led quality improvement projects (39%). Most PCPs perceived their closest champion as being moderately to extremely effective at improving vaccination rates (91%). PCPs who did versus did not work with champions more often recommended HPV vaccination at the earliest opportunity of ages 9–10 rather than later ages (44% vs. 33%, p < 0.001).
Conclusions
Findings of our national study suggest that vaccine champions are common in adolescent primary care, but only a minority lead quality improvement projects. Interventionists seeking to identify champions to improve HPV vaccination rates can expect to find them among both physicians and nurses, but should be prepared to offer support to more fully engage them in implementing interventions.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies.

- Record: found
- Abstract: found
- Article: found
A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project
- Record: found
- Abstract: found
- Article: not found