- Record: found
- Abstract: found
- Article: found
Postretinal Structure and Function in Severe Congenital Photoreceptor Blindness Caused by Mutations in the GUCY2D Gene
Read this article at
Abstract
Purpose
To examine how severe congenital blindness resulting from mutations of the GUCY2D gene alters brain structure and function, and to relate these findings to the notable preservation of retinal architecture in this form of Leber congenital amaurosis (LCA).
Methods
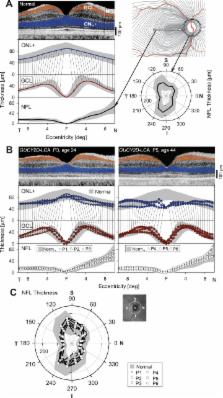
Six GUCY2D-LCA patients (ages 20–46) were studied with optical coherence tomography of the retina and multimodal magnetic resonance imaging (MRI) of the brain. Measurements from this group were compared to those obtained from populations of normally sighted controls and people with congenital blindness of a variety of causes.
Results
Patients with GUCY2D-LCA had preservation of the photoreceptors, ganglion cells, and nerve fiber layer. Despite this, visual function in these patients ranged from 20/160 acuity to no light perception, and functional MRI responses to light stimulation were attenuated and restricted. This severe visual impairment was reflected in substantial thickening of the gray matter layer of area V1, accompanied by an alteration of resting-state correlations within the occipital lobe, similar to a comparison group of congenitally blind people with structural damage to the retina. In contrast to the comparison blind population, however, the GUCY2D-LCA group had preservation of the size of the optic chiasm, and the fractional anisotropy of the optic radiations as measured with diffusion tensor imaging was also normal.
Related collections
Most cited references67
- Record: found
- Abstract: found
- Article: not found
A hybrid approach to the skull stripping problem in MRI.
- Record: found
- Abstract: found
- Article: not found
Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex.
- Record: found
- Abstract: found
- Article: not found