- Record: found
- Abstract: found
- Article: found
Knockdown of E2f1 by RNA interference impairs proliferation of rat cells in vitro
Read this article at
Abstract
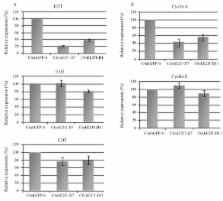
E2F1 plays a key role in cell-cycle regulation in mammals, since its transcription factor activity controls genes required for DNA synthesis and apoptosis. E2F1 deregulation is a common feature among different tumor types and can be a major cause of cell proliferation. Thus, blocking E2F1 expression by RNA interference represents a promising therapeutic approach. In this study, the introduction of specific short hairpin RNAs (shRNAs) reduced E2f1 expression by up to 77%, and impaired rat glioma cell proliferation by approximately 70%, as compared to control cells. Furthermore, we investigated the expression of E2f1 target genes, Cyclin A and Cyclin E. Cyclin A was found to be down-regulated, whereas Cyclin E had similar expression to control cells, indicating that gene(s) other than E2f1 control its transcription. Other E2f family members, E2f2 and E2f3, which have been classified in the same subgroup of transcriptional activators, were also analyzed. Expression of both E2f2 and E2f3 was similar to control cells, showing no cross-inactivation or up-regulation to compensate for the absence of E2f1. Nevertheless, their expression was insufficient to maintain the initial proliferation potential. Taken together, our results suggest that shE2f1 is a promising therapy to control tumor cell proliferation.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5.
- Record: found
- Abstract: found
- Article: not found
The E2F1-3 transcription factors are essential for cellular proliferation.
- Record: found
- Abstract: found
- Article: not found