- Record: found
- Abstract: found
- Article: found
Efficacy and safety of brodalumab, an anti-IL17RA monoclonal antibody, in patients with axial spondyloarthritis: 16-week results from a randomised, placebo-controlled, phase 3 trial
Read this article at
Abstract
Objective
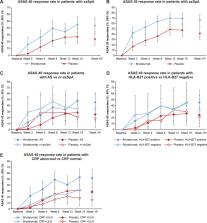
To investigate the efficacy and safety of brodalumab, a fully human anti-interleukin-17 receptor A monoclonal antibody, in patients with axial spondyloarthritis (axSpA).
Methods
In a multicentre, placebo-controlled phase 3 study (NCT02985983) conducted at 48 sites across Japan, Korea and Taiwan, patients with axSpA were randomised 1:1 to receive subcutaneous brodalumab 210 mg (n=80) or placebo (n=79) at baseline, weeks 1 and 2 and every 2 weeks thereafter, during the 16-week double-blind period. The primary endpoint was the proportion of patients with Assessment of SpondyloArthritis International Society (ASAS) 40 response at week 16. Secondary endpoints included the proportion of patients with ASAS 20 response and change in Ankylosing Spondylitis Disease Activity Score using C-reactive protein (ASDAS-CRP) at week 16 and safety.
Results
ASAS 40 response rate (n/N; 95% CI) was 43.8% (35/80; 32.7, 55.3) with brodalumab vs 24.1% (19/79; 15.1, 35.0) with placebo (rate difference, 19.7% (5.3, 34.1); p=0.018 by stratified Cochran-Mantel-Haenszel test). ASAS 20 response rate (n/N; 95% CI) was 67.5% (54/80; 56.1, 77.6) vs 41.8% (33/79; 30.8, 53.4) and least squares mean change (95% CI) from baseline (brodalumab, 2.660; placebo, 2.716) in ASDAS-CRP was –1.127 (–1.322, –0.931) with brodalumab vs –0.672 (–0.872, –0.473) with placebo at week 16. Treatment-emergent adverse events were reported in 44 (55%) and 45 (57%) patients in the brodalumab and placebo groups, respectively.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection.
- Record: found
- Abstract: found
- Article: found
2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis
- Record: found
- Abstract: found
- Article: not found