- Record: found
- Abstract: found
- Article: found
Differences in the Receptor Status between Primary and Recurrent Breast Cancer - The Frequency of and the Reasons for Discordance
Abstract
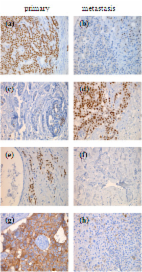
Objective: Receptor discordances between primary and recurrent breast cancer have been described for years, but only a few analyses have elucidated the factors that influence receptor changes. Methods: Explorative analyses of prospective data from a breast cancer database of a tertiary breast cancer unit. Results: Recurrent tumours that had expressed oestrogen (ER) and progesterone receptors (PR) and human epidermal growth factor receptor 2 (HER2) as primary tumours were negative for the respective receptor in 22.8, 41.4 and 40.8% of cases. ER, PR and HER2 expression was found in 19.8, 16.7 and 11.5% of recurrent tumours, although no expression had been observed in primary tumours. Receptor discordances in recurrent disease leading to different therapeutic approaches were noted in 126 of 411 patients (30.7%). In patients with tumours expressing primary ER and HER2, independent factors associated with discordance were endocrine therapy and treatment with trastuzumab. Conclusion: High rates of receptor discordance were found. The impact of factors that influence receptor changes is small so that no subgroup of patients with recurrent breast cancer should be excluded from biopsy. Whenever possible, a biopsy should be taken to confirm the diagnosis of a possible relapse as well as the receptor status of patients with breast cancer.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression.
- Record: found
- Abstract: found
- Article: not found
Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer.