- Record: found
- Abstract: found
- Article: found
Pathologic complete response and outcomes by intrinsic subtypes in NSABP B-41, a randomized neoadjuvant trial of chemotherapy with trastuzumab, lapatinib, or the combination
Read this article at
Abstract
Purpose
NSABP B-41, a phase three randomized trial, evaluated neoadjuvant lapatinib, trastuzumab, or the combination with chemotherapy in patients with HER2-positive operable breast cancer. Though no significant difference in pathologic complete response (pCR) was found among the three arms, pCR was associated with prolonged survival. We analyzed tumor intrinsic subtypes with Prediction Analysis of Microarray 50 in a subset of B-41 patients to determine their value in predicting HER2-targeting benefit.
Methods
Pearson’s Chi square test and logistic regression were used to compare pCR in the breast and nodes (ypT0/Tis ypN0). Kaplan–Meier estimates and Cox models were used to compare event-free and overall survival among subtypes.
Results
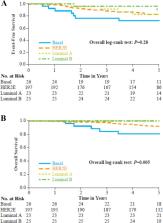
Intrinsic subtypes were determined in 271 baseline core biopsy samples. The pCR rate among patients with HER2-enriched (HER2E) subtype was greater compared to other subtypes combined (120/197, 60.9% versus 19/74, 25.7%; p < 0.001). In multivariate analysis among patients receiving trastuzumab-containing regimens (with clinical factors and HER2E subtype as factors), HER2E subtype was most strongly associated with pCR [OR 8.41 (95% CI 2.52–28.1) p < 0.001]. Patients with HER2E tumors did not benefit more from dual HER2-targeted therapy versus trastuzumab. The pCR rate was higher among HER2E tumors versus other subtypes in both estrogen receptor-positive and -negative tumors ( p ≤ 0.001). Higher ESR1 gene expression was associated with lower pCR rate. No association was observed between subtype and long-term outcomes.
Related collections
Most cited references15
- Record: found
- Abstract: not found
- Article: not found
Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing
- Record: found
- Abstract: found
- Article: not found
Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis.
- Record: found
- Abstract: not found
- Article: not found