- Record: found
- Abstract: found
- Article: found
Risk Factors Associated with Cognitive Decline after Cardiac Surgery: A Systematic Review
Read this article at
Abstract
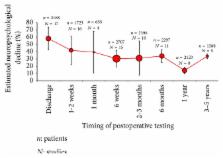
Modern day cardiac surgery evolved upon the advent of cardiopulmonary bypass machines (CPB) in the 1950s. Following this development, cardiac surgery in recent years has improved significantly. Despite such advances and the introduction of new technologies, neurological sequelae after cardiac surgery still exist. Ischaemic stroke, delirium, and cognitive impairment cause significant morbidity and mortality and unfortunately remain common complications. Postoperative cognitive decline (POCD) is believed to be associated with the presence of new ischaemic lesions originating from emboli entering the cerebral circulation during surgery. Cardiopulmonary bypass was thought to be the reason of POCD, but randomised controlled trials comparing with off-pump surgery show contradictory results. Attention has now turned to the growing evidence that perioperative risk factors, as well as patient-related risk factors, play an important role in early and late POCD. Clearly, identifying the mechanism of POCD is challenging. The purpose of this systematic review is to discuss the literature that has investigated patient and perioperative risk factors to better understand the magnitude of the risk factors associated with POCD after cardiac surgery.
Related collections
Most cited references106
- Record: found
- Abstract: found
- Article: not found
Long-term consequences of postoperative cognitive dysfunction.
- Record: found
- Abstract: found
- Article: not found
Role of interleukin-1beta in postoperative cognitive dysfunction.
- Record: found
- Abstract: found
- Article: not found