- Record: found
- Abstract: found
- Article: found
A hydrophobic gate in the inner pore helix is the major determinant of inactivation in mechanosensitive Piezo channels
Read this article at
Abstract
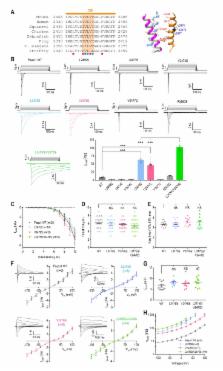
Piezo1 and Piezo2 belong to a family of mechanically-activated ion channels implicated in a wide range of physiological processes. Mechanical stimulation triggers Piezo channels to open, but their characteristic fast inactivation process results in rapid closure. Several disease-causing mutations in Piezo1 alter the rate of inactivation, highlighting the importance of inactivation to the normal function of this channel. However, despite the structural identification of two physical constrictions within the closed pore, the mechanism of inactivation remains unknown. Here we identify a functionally conserved inactivation gate in the pore-lining inner helix of mouse Piezo1 and Piezo2 that is distinct from the two constrictions. We show that this gate controls the majority of Piezo1 inactivation via a hydrophobic mechanism and that one of the physical constrictions acts as a secondary gate. Our results suggest that, unlike other rapidly inactivating ion channels, a hydrophobic barrier gives rise to fast inactivation in Piezo channels.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Piezos are pore-forming subunits of mechanically activated channels
- Record: found
- Abstract: found
- Article: not found
Piezo1, a mechanically activated ion channel, is required for vascular development in mice.
- Record: found
- Abstract: found
- Article: not found