- Record: found
- Abstract: found
- Article: found
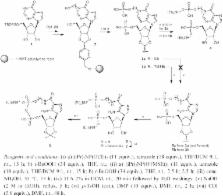
Solid-Phase Synthesis of a New Diphosphate 5-Aminoimidazole-4-carboxamide Riboside (AICAR) Derivative and Studies toward Cyclic AICAR Diphosphate Ribose
research-article
Stefano D’Errico
1 ,
Giorgia Oliviero
1
,
2
,
* ,
Nicola Borbone
1 ,
Jussara Amato
1 ,
Vincenzo Piccialli
3 ,
Michela Varra
1 ,
Luciano Mayol
1 ,
Gennaro Piccialli
1
,
2
21 September 2011
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
The solid-phase synthesis of the first example of a new diphosphate AICAR derivative is reported. The new substance is characterized by the presence of a 5'-phosphate group while a second phosphate moiety is installed on a 5-hydroxypentyl chain attached to the 4- N-position of AICAR. Cyclization of the diphosphate derivative by pyrophosphate bond formation allowed for the formation of a novel AICAR-based cyclic ADP-ribose (cADPR) mimic.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Roles of 5'-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis.

- Record: found
- Abstract: found
- Article: found
AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease
Aaron K. F. Wong, Jacqueline Howie, John R Petrie … (2009)
- Record: found
- Abstract: found
- Article: not found
Pyrimidine as constituent of natural biologically active compounds.
Irene?M. Lagoja (2005)