- Record: found
- Abstract: found
- Article: not found
Biology and Diseases of Rabbits
chapter-article
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
I.
INTRODUCTION
Rabbits have been used extensively in a variety of biomedical research disciplines.
The need for consistent research subjects has led to understanding of the basic biology
and special needs of rabbits. This chapter will provide a summary of care, management,
and diseases of the laboratory rabbit.
It is ironic that while effort is given to promote the health of domestic rabbits,
feral populations have the ability to explode to plague proportions in areas of the
world where natural predators and diseases are limited. In 1890, the rabbit population
of Australia was estimated at 20 million. All of these individuals originated with
one pair of rabbits introduced to the continent 31 years previously (Fox, 1994). It
is further ironic that while effort is given to control infectious pathogens of domestic
rabbits, in other circumstances such agents have been used to control feral populations.
For example, myxoma virus has been used to control overpopulation of wild rabbits
(DiGiacomo and Maré, 1994). Finally, although not intentionally released, the calicivirus
agent for rabbit hemorrhagic disease may have killed up to 30 million rabbits during
a 1-month epidemic at Flinders Ranges National Park in Australia (Mutze et al., 1998).
This outbreak is responsible for the death of 2.6 times as many rabbits as those used
in biomedical research in the United States from 1973 through 1997.
A.
Taxonomy
The terms “rabbit” and “hare” are often misused when referring to common names or
breeds of rabbits (Fox, 1994; Nowak and Paradiso, 1983). Animals classified in the
genus Lepus are the only true hares. There are several genera that contain rabbits.
Oryctolagus cuniculus is the only domesticated rabbit, and consequently the only species
from which unique breeds are derived.
Many breeds have been developed simply by selective breeding of O. cuniculus for different
physical characteristics. Currently, 42 breeds are recognized by the American Rabbit
Breeders Association. A list of these breeds is found in Table I
. In addition to those described in Table I, over 100 different gene mutations have
been described, and these phenotypes are used for the study of human disease. The
inheritance properties of these mutations are described in detail elsewhere (Fox,
1994).
Table I
Breeds of Rabbits Recognized by the American Rabbit Breeders Associationa
American Blue & White
Havana
American Checkered Giant
Himalayan
American Chinchilla
Holland Lop
American Dutch
Hotot
American Sable
Jersey Wooly
Angora
Lilac
Belgian Hare
Lop
Beverens
Mini Lop
Britannia Petite
Mini Rex
Californian
Netherland Dwarf
Cavy
New Zealand
Champagne d'Argent
Palomino
Cinnamon
Polish
Creme d'Argent
Rex
Dwarf Hotot
Rhinelander
English Spot
Satin
Flemish Giant
Silver Fox
Florida White
Silver Marten
Fuzzy Lop
Silver
Giant Chinchilla
Standard Chinchilla
Harlequin
Tan
a
Despite the different breed names and the use of the word hare for some breeds, all
are derived from Oryctolagus cuniculus.
The following list shows the complete taxonomic position of animals in the order Lagomorpha.
Class: Mammalia
Order: Lagomorpha
Family: Ochotonidae (pikas)
Genus: Ochotona
Species: 19 species
Family: Leporidae (rabbits and hares)
Subfamily: Leporinae
Genus/Species:
Bunolagus monticularis (Bushman rabbit)
Brachylagus idahoensis (Idaho pygmy rabbit)
Caprolagus hispidus (hispid hare)
Lepus, 22 species (“true” hares, jackrabbits)
Nesolagus netscheri (Sumatra short-eared rabbit)
Oryctolagus cuniculus (European rabbit,
Old World rabbit)
Pentalagus furnessi (Amami rabbit)
Poelagus marjorita (Bunyoro rabbit)
Pronolagus, 3 species (rock hare)
Romerolagus diazzi (volcano rabbit)
Sylvilagus, 14 species (cottontail rabbits)
B.
Use in Research
Since 1973, the U.S. Department of Agriculture has reported the total number of certain
species of animals used by registered research facilities (Animal and Plant Health
Inspection Service, 1997). Table II
indicates the total number of rabbits used in research as reported to the USDA for
the period 1973–1997. Despite the overall drop in the number used in research, the
rabbit is still a valuable model and tool for many disciplines. It is not a goal of
this chapter to discuss in detail the different research uses of the rabbit. Rather,
a few broad comments and examples of rabbit use will be offered.
Table II
Numbers of Rabbits Used in Biomedical Research in the United States, 1973–1997a
1973
447,570
1974
425,585
1975
448,530
1976
527,551
1977
439,003
1978
475,162
1979
539,594
1980
471,297
1981
473,922
1982
453,506
1983
466,810
1984
529,101
1985
544,621
1986
521,773
1987
554,385
1988
459,254
1989
471,037
1990
399,264
1991
396,046
1992
431,432
1993
426,501
1994
393,751
1995
354,076
1996
338,574
1997
309,322
a
Total number of rabbits used in research as reported to the U.S. Department of Agriculture.
Notice the trend toward reduced use of rabbits over the course of 25 years.
One of the most common research uses of rabbits is in the production of polyclonal
antibodies. The relatively large body size and blood volume, easy access to the vascular
system, and an existent large body of information on the purification of rabbit immunoglobulins
are a few reasons the rabbit is preferred over other common laboratory animal species
for polyclonal antibody production (Stills, 1994).
The Armed Forces Institute of Pathology (AFIP) has recognized at least 22 different
spontaneous or induced diseases of the rabbit that are models of human diseases. Half
of these models can be grouped into two categories: cancer and infectious agent models.
Other recognized rabbit models of human disease include hydrocephalus induced by vitamin
A deficiency (Newberne, 1974); hypervitaminosis A (Shenefelt, 1972); acute respiratory
distress syndrome induced by phorbol myristate acetate (Salzer and McCall, 1991);
diabetes mellitus (Roth and Conaway, 1983); inflammatory bowel disease (Rabin, 1980);
methylmercury poisoning (Koller, 1979); and the Pelger–Huët anomaly (Tvedten, 1983).
There are six cancer models listed by the AFIP. The VX-2 tumor, spontaneous endometrial
adenocarcinoma, monoclonal gammopathies, nephroblastoma, lymphoblastic leukemia, and
malignant fibroma are all considered animal models of human neoplastic disease. The
VX-2 carcinoma results from the malignant transformation of the viral-induced Shope
papilloma. The tumor induces fulminating hypercalcemia, within 4 weeks of implantation
(Young et al., 1978). Endometrial adenocarcinoma is most common in aged rabbits, with
an incidence of 79% being reported in a colony of 5-year-old rabbits (Baba and von
Haam, 1972). The other rabbit models of neoplasia described above are induced models.
Monoclonal gammopathies can be induced in the rabbit in response to specific bacterial
components (Hurvitz, 1975). Nephroblastoma is induced by administration of ethylnitrosourea
to pregnant does (Haenichen and Stavrou, 1980). Finally, transgenic technology has
been utilized to create a transgenic rabbit that develops acute β-lymphoblastic leukemia
as a weanling (Sethupathi et al., 1993).
The rabbit has been used extensively for infectious disease research, such as studies
on Campylobacter enteritis (Caldwell and Walker, 1986), Chagas’ disease (Texeira,
1986), cryptococcal meningitis (Perfect, 1985), Herpes simplex encephalitis (Schlitt
and Bucher, 1989), and staphylococcal blepharitis (Mondino and Phinney, 1989).
Another area in which the rabbit has been frequently employed as a model is in work
related to cardiovascular disease. Numerous dietary modifications will induce or exacerbate
cholesterol-induced atherosclerosis in the rabbit. A brief overview of some of these
dietary modifications can be found elsewhere (Jayo et al., 1994).
Research efforts into cholesterol metabolism have used the Watanabe heritable hyperlipidemic
(WHHL) (Atkinson et al., 1992; Kita et al., 1981) and the St. Thomas Hospital strain
rabbits (LaVille et al., 1987). The WHHL rabbit has a marked deficiency of low-density
lipoprotein (LDL) receptors in the liver and other tissues. Selective breeding of
the WHHL rabbit will increase the incidence of coronary artery atherosclerosis without
increasing the incidence of aortic atherosclerosis (Watanabe et al., 1985). In contrast,
the St. Thomas Hospital strain has a normal functioning LDL receptor but still maintains
a hypercholesterolemic state (LaVille et al., 1987).
II.
BIOLOGY
A.
Comparative Anatomy and Physiology
1.
Digestive System
The mouth of the rabbit is relatively small, and the oral cavity and pharynx are long
and narrow. The dental formula is i2/1,c0/0,pm3/2,m2–3/3 × 2 = 26 or 28 teeth.
A small pair of incisors is present directly caudal to the primary maxillary incisors
and is referred to as the “peg” teeth. The peg teeth are used along with the primary
incisors to bite and shear food. The absence of second incisors has been noted in
some rabbit herds as a dominant trait (I2/I2 or I2/i2). The teeth of rabbits erupt
continuously throughout life and therefore will continue to grow and lengthen unless
normal occlusion and use are sufficient to wear teeth to a normal length. Molars do
not have roots and are characterized by deep enamel folds. Rabbits normally masticate
food with a chewing motion that facilitates grinding of food by movement of the premolars
and molars from side to side and front to back.
The rabbit has four pairs of salivary glands, including the parotid, submaxillary,
sublingual, and zygomatic. The parotid is the largest and lies laterally just below
the base of the ear. The zygomatic salivary gland does not have a counterpart in humans.
The esophagus of the rabbit has three layers of striated muscle that extend the length
of the esophagus down to, and including, the cardia of the stomach. This is in contrast
to humans and many other species of animals, who have separate portions of striated
and smooth muscle along the length of the esophagus. There are no mucous glands in
the esophagus of the rabbit.
Although the stomach of the rabbit holds approximately 15% of the volume of the gastrointestinal
tract, it is never entirely empty in the healthy rabbit. The gastric contents often
include a large amount of hair ingested as the result of normal grooming activity.
The stomach is divided into the cardia, fundus, and pylorus.
The liver has four lobes. The gallbladder is found located on the right. From the
liver, the common bile duct empties into the duodenum posterior to the pylorus. Rabbits
produce relatively large amounts of bile compared to other common species. The pancreas
is diffuse within the mesentery of the small intestine and enters the duodenum 30
to 40 cm distal to the common bile duct.
The small intestine of the rabbit is short relative to that of other species and comprises
approximately 12% of the total length of the gastrointestinal (GI) tract. Because
the GI tract of the rabbit is relatively impermeable to large molecules, kits receive
most of their passive immunity via the yolk sac prior to birth rather than by the
colostrum. Pale foci of lymphoid tissue referred to as Peyer's patches are found along
the ileum, particularly near the cecal junction. The sacculus rotundus is a large
bulb of lymphoid tissue located at this junction.
The large intestine includes the cecum, the ascending colon, the transverse colon,
and the descending colon. The ileocecal valve regulates flow of chyme into the cecum
and retards reverse flow back into the ileum. The cecum is very large with a capacity
approximately 10 times that of the stomach. The cecum ends in a blind sac, the appendix.
The colon is divided into proximal and distal portions by the fusus coli, which serves
to regulate the elimination of hard versus soft fecal pellets. Hard pellets comprise
about two-thirds of the fecal output. Soft pellets, or “cecotrophs,” have a high moisture
content and are rich in nitrogen-containing compounds (Ferrando et al., 1970) and
the B vitamins niacin, riboflavin, pantothenate, and cyanocobalamin. Rabbits consume
cecotrophs directly from the anus to obtain significant nutritional benefit. Soft
pellets are sometimes termed “night feces,” since they are generally produced at night
in domestic rabbits (Fig. 1
). In contrast, the circadian rhythm of cecotrophy is reversed in wild rabbits, occurring
during the day when the animals are in their burrows (Hornicke, 1977).
Fig. 1
Normal stomach contents from a rabbit. Note the smooth, round mucoid night feces along
with the amorphous food mass. Night feces are thought to originate from the cecum
and are usually passed during the night and consumed by the rabbit. The night feces
are easily distinguished from the discrete oval fecal pellets produced during the
day.
2.
Respiratory System
Nostrils of rabbits are well equipped with touch cells, and they have a well-developed
sense of smell. Nasal breathing in rabbits is characterized by twitching of the nostrils
at rates varying from 20 to 120 times per minute, although twitching may be absent
in the relaxed rabbit. It has been speculated that inspiration occurs as the nostril
moves up and that this serves to direct the flow of air over the turbinate bones where
the olfactory cells are most concentrated.
The musculature of the thoracic wall contributes little to respiratory efforts. Instead,
rabbits rely mostly on the activity of the diaphragm. Because of this, artificial
respiration is easily performed by alternating the head of the rabbit between the
up position and the down position, 30–45 times per minute, while holding the animal.
Compression and release of the chest wall is an ineffective means of artificial respiration
in the rabbit.
The pharynx of the rabbit is long and narrow, and the tongue is relatively large.
These features make endotracheal intubation difficult to perform in the rabbit. The
procedure is further complicated by the propensity of the rabbit to laryngospasm during
attempts to intubate the trachea.
The rabbit lungs consist of six lobes. Both right and left sides have cranial, middle,
and caudal lobes, with the right caudal being further subdivided into lateral and
medial portions. Flow volume of air to the left lung is higher than to the right due
to the lower resistance of the proximal airways per unit volume (Yokoyama, 1979).
In rabbits, lung volume increases with age, in contrast to that of humans and dogs,
in which it decreases. Bronchial-associated lymphoid tissue (BALT) is present as distinct
tissue.
3.
Cardiovascular System
A unique feature of the cardiovascular system of the rabbit is that the tricuspid
valve of the heart has only two cusps, rather than three as in many other mammals.
A small group of pacemaker cells generates the impulse of the sinoatrial (SA) node
in the rabbit, a feature that facilitates precise determination of the location of
the pacemaker (Bleeker et al., 1980; Hoffmann, 1965; West, 1955). The SA and atrioventricular
(AV) nodes are slender and elongated, and the AV node is separated from the annulus
fibrosus by a layer of fat (Truex and Smythe, 1965).
Additional unique anatomic features of the cardiovascular system of the rabbit have
been utilized to advantage. The aortic nerve subserves no known chemoreceptors (Kardon
et al., 1974; Stinnett and Sepe, 1979) and responds to baroreceptors only. Because
the aortic nerve, which becomes the depressor nerve, runs alongside but separate from
the vagosympathetic trank, it lends itself readily to implantation of electrodes (Karemaker
et al., 1980).
The blood supply to the brain is restricted mainly to the internal carotid artery.
Blood supplied via the vertebral arteries is limited. The aorta of the rabbit demonstrates
rhythmic contractions that arise from neurogenic stimulation in a pattern related
to the pulse wave (Mangel et al., 1981).
4.
Urogenital System
The kidney of the rabbit is unipapillate in contrast to that of most other mammals,
which is multipapillate. This feature increases the ease with which cannulization
is performed. The right kidney lies more cranial than the left.
Glomeruli increase in number after birth, whereas all of the glomeruli are present
at birth in humans (Smith, 1951). Ectopic glomeruli are normal in the rabbit (Steinhausen
et al., 1990). Blood vessels that perfuse the medulla remain open during many conditions
under which vasoconstriction of the cortical tissue occurs; thus, the medullary tissue
may be perfused while the cortex is ischemic (Trueta et al., 1947).
In the rabbit, the clearance of creatinine is identical with the clearance of insulin,
thus creatinine clearance can be used to accurately measure the glomerular filtration
rate. This is not true for primates, rats, or guinea pigs, among others.
The urine of adult rabbits is typically cloudy due to a relatively high concentration
of ammonium magnesium phosphate and calcium carbonate monohydrate precipitates (Flatt
and Carpenter, 1971). The urine may also take on hues ranging from yellow or reddish
to brown. In contrast, the urine of young rabbits is typically clear, although healthy
young rabbits may have albuminuria. The urine is normally yellow but can also take
on reddish or brown hues once they begin to eat green feed and cereal grains. Normal
rabbits have few cells, bacteria, or casts in their urine. The pH of the urine is
typically alkaline at about 8.2 (Williams, 1976). A normal adult rabbit produces approximately
50–75 ml/kg of urine daily (Gillett, 1994), with does urinating more copiously than
bucks.
The urethral orifice of the buck is rounded, whereas that of the doe is slitlike.
This feature is useful for distinguishing the sexes. The testes of the adult male
usually lie within the scrotum; however, the inguinal canals that connect the abdominal
cavity to the inguinal pouches do not close in the rabbit. For this reason, the testes
can easily pass between the scrotum and the abdominal cavity. In particular, this
feature necessitates closure of the superficial inguinal ring following orchiectomy
by open technique, to prevent herniation.
The reproductive tract of the doe is characterized by two uterine horns that are connected
to the vagina by separate cervices (bicornuate uterus) (Fig. 2
). A common tube, the urogenital sinus or vestibulum, is present where the urethra
enters the vagina. The placenta is hemochorial, and maternal blood flows into sinuslike
spaces where the transfer of nutrients and other substances to the fetal circulation
occurs (Jones and Hunt, 1983).
Fig. 2
Rabbit uterus. Note two uterine horns each with its own cervix (arrows).
Inguinal pouches are located lateral to the genitalia in both sexes. The pouches are
blind and contain scent glands that produce white to brown secretions that may accumulate
in the pouch.
5.
Metabolism
The metabolic rate of endotherms is generally related to the body surface area. Including
the ears, the rabbit has a relatively low metabolic rate (MR); however, if the surface
area of the ears is discounted, the MR of the rabbit is similar to that of other endotherms.
Neonatal rabbits have an amount of body fat comparable to that of the human infant
(16% of body weight) (Cornblath and Schwartz, 1976). The neonatal rabbit is essentially
an ectotherm until about day 7 (Gelineo, 1964). The glucose reserves of the neonatal
rabbit are quickly depleted, usually within about 6 hr after birth (Shelley, 1961).
The fasting neonatal rabbit quickly becomes hypoglycemic and ketotic (Callikan and
Girard, 1979).
The normal rectal temperature of the adult New Zealand White rabbit at rest is approximately
38.5° to 39.5°C (Ruckebusch et al., 1991). The ears serve an important thermoregulatory
function. Because they have a large surface area and are highly vascular with an extensive
arteriovenous anastomotic system, the ears help the rabbit sense and respond to cold
versus warm temperatures (Kluger et al., 1972). In addition, the ears serve as a countercurrent
heat-exchange system to help adjust body temperature.
Early studies found that the body of the adult rabbit (3 kg body weight) consists
of greater than 50% water (58%), with a half-time turnover of about 3.9 days and a
loss of about 340 ml daily (Richmond et al., 1962). The amount of water ingested varies
with the amount and type of feed consumed and the environmental temperature. In general,
rabbits will drink more water when consuming dry, pelleted feed than when consuming
foodstuffs high in moisture, such as fresh greens. Conversely, rabbits deprived of
water will decrease food consumption. After 3 days of complete water deprivation,
the food intake falls to less than 2% of normal (Cizek, 1961).
B.
Normative Physiological Values
Normal values for various systems and parameters are provided as a general indication
for these values in the rabbit. It is important to recognize, however, that most of
these values have been obtained through the study of adult New Zealand White rabbits.
Values can vary significantly between breeds, laboratories, methods of sampling and
measurement, and individual rabbits due to age, sex, breed, health, handling, and
husbandry (Hewitt et al., 1989; Lidena and Trautschold, 1986; Mitruka and Rawnsley,
1981; Woolford et al., 1986; Yu et al., 1979). For this reason, individual laboratories
should strive to establish their own normal values, whenever possible.
1.
Hematologic Values
Values for hematologic parameters are shown in Table III
. These values represent those typical of adult New Zealand White rabbits. In general,
males have slightly greater hematocrit and hemoglobin values than females (Mitruka
and Rawnsley, 1981).
Table III
Hematologic Values for the Adult Rabbita
Hematologic parameter
Typical value
Blood volume
55–65 ml/kg
Plasma volume
28–50 ml/kg
Hemoglobin
9.8–14.0 gm/dl
Packed cell volume
34–43%
Erythrocytes
5.3–6.8 cells (106/μl)
Reticulocytes
1.9–3.8%
Mean corpuscular volume (MCV)
60–69A
Mean corpuscular hemoglobin (MCH)
20–23 pg
MCH concentration (MCHC)
31–35%
Sedimentation rate
0.92–3.00 mm/hr
White blood cells
5.1–9.7 cells (103/μl)
Neutrophils (heterophils)
25–46%
Lymphocytes
39–68%
Eosinophils
0.1–2.0%
Basophils
2.0–5.0%
Monocytes
1.0–9.0%
Platelets
158–650 (103/μl)
a
Values obtained from the following sources: Burns and DeLannoy (1966), Gillett (1994),
Kabata etal. (1991), Mitruka and Rawnsley (1981), and Woolford et al. (1986).
Red blood cell (RBC) diameter reaches normal adult values of 6.7–7.9 mm (Jain, 1986).
Anisocytosis is normal and accounts for variation in reported values for RBC diameter
(Sanderson and Phillips, 1981). The life span of the rabbit RBC averages 57 days although
some could survive up to 67 days (Vacha, 1983). Reticulocyte values are usually between
2% and 4% in healthy rabbits (Corash et al., 1988). Red blood cell sedimentation is
minimal, with values of 1–3 mm/hr being typical (Schermer, 1967). Platelets have a
pale blue cytoplasm and azurophilic granules when stained by standard methods (Jain,
1986; Sanderson and Phillips, 1981). The neutrophil of the rabbit is sometimes referred
to as a “pseudoeosinophil” or “heterophil,” due to the presence of red-staining granules
in the cytoplasm. The heterophil (10–15 mm in diameter) is, however, smaller than
the eosinophil (12–16 mm in diameter) (Sanderson and Phillips, 1981). In addition,
the red granules of the heterophil are smaller than the red granules of the eosinophil.
The nucleus of the eosinophil may be either bilobed or horseshoe-shaped.
Some rabbits demonstrate the Pelger-Huët anomaly in which the heterophil nucleus is
hyposegmented due to incomplete differentiation of the granulocytes (Jain, 1986).
Although the typical presentation is that of a few Pelger cells in the circulation,
one report describes a line of rabbits with uniform presence of Pelger cells in the
circulation accompanied by high mortality (Schermer, 1967).
The morphology of lymphocytes and monocytes is similar to that seen in other mammals.
Both small (7–10 μm in diameter) and large (10–15 μm in diameter) lymphocytes are
typically present (Jain, 1986; Sanderson and Phillips, 1981). The largest cell in
the peripheral blood circulation of the rabbit is the monocyte, at 15–18 μm in diameter.
Granules are not normally found in the cytoplasm of rabbit monocytes.
2.
Blood and Serum Chemistry and Enzyme Values
As mentioned earlier, chemistry values can vary because of a number of factors. For
this reason, each laboratory should establish its own normal values.
Aspartate aminotransferase (AST), formerly serum glutamate oxalate transaminase (SGOT),
is present in the liver, heart, skeletal muscle, kidney, and pancreas. Collection
of blood samples in rabbits by decapitation, cardiac puncture, or aortic incision,
or the use of restraint that causes exertion will elevate AST levels due to muscle
damage (Lidena and Trautschold, 1986). Similarly, levels of creatinine kinase are
sensitive to muscle damage since that enzyme is present in skeletal muscle, brain,
and heart (Lidena and Trautschold, 1986; Mitruka and Rawnsely, 1981).
Although most mammals have two isoenzymes (intestinal and a liver/kidney/bone form)
of alkaline phosphatase (AP), rabbits are unique in having three forms of AP, including
an intestinal form and two forms that are both present in the liver and the kidney
(Noguchi and Yamashita, 1987). Values for blood and serum chemistry are shown in Table
IV
.
Table IV
Values of Serum Biochemical and Enzyme Parameters of the Adult Rabbita
Biochemical parameter
Typical value
Total protein
5.0–7.5 gm/dl
Globulin
1.5–2.7 gm/dl
Albumin
2.7–5.0 gm/dl
Glucose
74–148 mg/dl
Sodium
125–150 mEq/liter
Chloride
92–120mEq/liter
Potassium
3.5–7.0 mEq/liter
Phosphorus
4.0–6.0 mg/dl
Calcium
5.60–12.1 mg/dl
Magnesium
2.0–5.4 mg/dl
Acid phosphatase
0.3–2.7 IU/liter
Alkaline phosphatase
10–86IU/liter
Acid phosphatase
0.30–2.70 IU/liter
Lactate dehydrogenase
33.5–129 IU/liter
γ-Glutamyltransferase
10–98 IU/liter
Aspartate aminotransferase
20–120 IU/liter
Creatine kinase
25–120 IU/liter
Alanine aminotransferase (SGPT)
25–65 IU/liter
Sorbitol dehydrogenase
170–177U
Urea nitrogen
5–25 mg/dl
Creatinine
0.5–2.6 mg/dl
Total bilirubin
0.2–0.5 mg/dl
Uric acid
1.0–4.3 mg/dl
Amylase
200–500 IU/liter
Serum lipids
150–400 mg/dl
Phospholipids
40–140 mg/dl
Triglycerides
50–200 mg/dl
Cholesterol
10–100 mg/dl
Corticosterone
1.54 μg/dl
a
Values obtained from the following sources: Burns and DeLannoy (1966), Fox (1989),
Gillett (1994), Kraus et al. (1984), and Loeb and Quimby (1989).
3.
Respiratory, Circulatory and Miscellaneous Biologic Parameters
Cardiovascular and respiratory function are often altered with experimental manipulation,
anesthesia, or disease. Normal values for these parameters and other miscellaneous
biologic characteristics of the rabbit are shown in Table V
.
Table V
Respiratory, Circulatory, and Miscellaneous Biologic Parameters of the Rabbita
Parameter
Typical value
Life span
5–7 years
Body weight
2–5 kg
GI transit time
4–5hr
Number of mammary glands
8 or 10
Diploid chromosome number
44
Body temperature
38.5°-39.5°C
Respiratory rate
32–60 breaths/min
Lung weight (2.4 kg rabbit)
9.1 gm
Total lung capacity
111 ± 14.7ml
Minute volume
0.6 liter/min
Tidal volume
4–6 ml/kg body weight
Mean alveolar diameter
93.97 μηι
Heart rate
200–300beats/min
po2
85–102 mmHg
pCo2
20–46 torr
HCo3
12–24 mmol/liter
Arterial oxygen
12.6–15.8% volume
Arterial systolic pressure
90–130 mmHg
Arterial diastolic pressure
80–90 mmHg
Arterial blood pH
7.2–7.5
Interstitial fluid (IF) colloid osmotic pressure
13.6mmHg
IF viscosity (water = 1)
1.9
IF protein
2.7
Cerebrospinal fluid (CSF) white blood cells
0–7 cells/mm3
CSF lymphocytes
40–79%
CSF monocytes
21–60%
a
Values obtained from the following sources: Barzago et al. (1992), Curiel et al. (1982),
Gillett (1994), Kozma et al. (1974), Sanford and Colby (1980), Suckow and Douglas
(1997), and Zurovsky et al. (1995).
C.
Nutrition
Rabbits are strictly herbivorous with a preferred diet of herbage that is low in fiber
and high in protein and soluble carbohydrate (Cheeke, 1987, 1994). Rabbits will generally
accept a pelleted feed more readily than one in meal form. When a meal diet is needed,
a period of adjustment should be allowed for the rabbits to accommodate to the new
diet. Examples of adequate diets are shown in Table VI
.
Table VI
Examples of Adequate Diets for Commercial Productiona
Kind of animal
Ingredients
Percentage of total dietb
Growth, 0.5–4 kg
Alfalfa hay
50.00
Corn, grain
23.50
Barley, grain
11.00
Wheat bran
5.00
Soybean meal
10.00
Salt
0.50
Maintenance, does and bucks, average 4.5 kg
Clover hay
70.00
Oats, grain
29.50
Salt
0.50
Pregnant does, average 4.5 kg
Alfalfa hay
50.00
Oats, grain
45.50
Soybean meal
4.00
Salt
0.50
Lactating does, average 4.5 kg
Alfalfa hay
40.00
Wheat, grain
25.00
Sorghum grain
22.50
Soybean meal
12.00
Salt
0.50
a
From Subcommittee on Rabbit Nutrition (1977). Used with permission.
b
Composition given on an as-fed basis.
The exact nutrient requirements for individual rabbits vary with age, reproductive
status, and health of the animal. Nutritional requirements for the domestic rabbit
are shown in Table VII
. On occasion, the need arises for use of highly purified diets. A suggested purified
diet has been described elsewhere (Subcommittee on Rabbit Nutrition, 1977). It should
be noted that overfeeding of laboratory rabbits resulting in obesity is common, but
can be prevented by either reducing the amount of feed or by providing a low-energy,
high-fiber maintenance diet.
Table VII
Nutrient Requirements of Rabbits Fed ad libitum (Percentage or Amount per Kilogram
of Diet)a
,
b
Nutrients
Growth
Maintenance
Gestation
Lactation
Energy and protein
Digestible energy (kcal)
2500.00
2100.00
2500.00
2500.00
Total digestible nutrients (%)
65.00
55.00
58.00
70.00
Crude fiber (%)
10–12c
14c
10–12c
10–12c
Fat (%)
2c
2
c
2c
2
c
Crude protein (%)
16.00
12.00
15.00
17.00
Inorganic nutrients
Calcium (%)
0.4
—d
0.45c
0.75c
Phosphorus (%)
0.22
—d
0.37c
0.5
Magnesium (mg)
300–400
300–400
300–400
300–400
Potassium (%)
0.6
0.6
0.6
0.6
Sodium (%)
0.2c
,
e
0.2c
,
e
0.2c
,
e
0.2c
,
e
Chlorine (%)
0.3c
,
e
0.3c
,
e
0.3c
,
e
0.3c
,
e
Copper (mg)
3
3
3
3
Iodine (mg)
0.2c
0.2c
0.2c
0.2c
Iron
−f
−f
−f
—d
Manganese (mg)
8.5f
2.5f
2.5f
2.5f
Zinc
—d
—d
—d
—d
Vitamins
Vitamin A (IU)
580
—d
> 1160
—d
Vitamin A as carotene (mg)
0.83c
,
d
—g
0.83c
,
d
—g
Vitamin D
—h
—h
—h
—h
Vitamin E (mg)
40i
−f
40h
40i
Vitamin K (mg)
—j
—j
0.2c
—j
Niacin (mg)
180
—k
—k
—k
Pyridoxine (mg)
39
—k
—k
—k
Choline (gm)
1.2c
—k
—k
—k
Amino acids (%)
Lysine
0.65
—h
—h
—h
Methionine + cystine
0.6
—h
—h
—h
Arginine
0.6
—h
—h
—h
Histidine
0.3c
—h
—h
—h
Leucine
1.1c
—h
—h
—h
Isoleucine
0.6c
—h
—h
—h
Phenylalanine + tyrosine
l.lc
—h
—h
—h
Threonine
0.6c
—h
—h
—h
Tryptophan
0.2c
—h
—h
—h
Valine
0.7c
—h
—h
—h
Glycine
—h
—h
—h
—h
a
From Subcommittee on Rabbit Nutrition (1977). Used with permission.
b
Nutrients not listed indicate dietary need unknown or not demonstrated.
c
May not be minimum but known to be adequate.
d
Quantitative requirement not determined, but dietary need demonstrated.
e
May be met with 5% NaCl.
f
Converted from amount per rabbit per day using an air-dry feed intake of 60 gm per
day for a 1-kg rabbit.
g
Quantitative requirement not determined.
h
Probably required; amount unknown.
i
Estimated.
j
Intestinal synthesis probably adequate.
k
Dietary need unknown.
As mentioned earlier, rabbits engage in cecotrophy, and by doing so supplement their
supply of protein and B vitamins. Rabbits fed a diet high in fiber ingest a greater
quantity of cecotropes than those on a lower-fiber diet (Fekete and Bokori, 1985).
Prolonged feeding of diets high in calcium, such as those with a high level of alfalfa
meal, can result in renal disease. Consumption of diets containing excessive vitamin
D can result in calcification of soft tissues, including the liver, kidney, vasculature,
and muscles (Fig. 3
) (Besch-Williford et al., 1985).
Fig. 3
Calcified aorta resulting from excessive dietary Vitamin D.
Diets that are either too high or too low in vitamin A can result in reproductive
dysfunction and congenital hydrocephalus (Cheeke, 1987; DiGiacomo et al., 1992). The
exact requirement for vitamin A in the rabbit has not been determined; however, a
level of 10,000 IU/kg of diet is generally adequate.
Vitamin E deficiency has been associated with infertility, muscular dystrophy, fetal
death, neonatal death, and colobomatous microphthalmos in rabbits (Nielsen and Carlton,
1995; Ringler and Abrams, 1970, 1971). McDowell (1989) suggests that serum vitamin
E levels of less than 0.5 μg/ml are indicative of hypovitaminosis E.
Relative to other species, rabbits have a high water intake. In general, daily water
intake is approximately 120 ml per kilogram of body weight. Consumption of water is
influenced by environmental temperature, disease states, and feed composition and
intake (Cizek, 1961). Consumption of diets high in fiber usually result in increased
water intake. Water consumption also increases with food deprivation.
D.
Behavior
Rabbits are social animals and attempts at group housing often meet with success,
although mature males will fight and can inflict serious injury on one another (Love,
1994; Podberscek et al., 1991; Whary et al., 1993). Group-penned female rabbits allowed
to choose between single or paired housing prefer being in the same cage with other
rabbits (Huls et al., 1991). In general, rabbits are timid and nonaggressive. Some
animals will display defensive behavior, typically characterized by thumping the cage
floor with the rear feet, biting, and charging toward the front of the cage when opened.
Laboratory-housed rabbits demonstrate diurnal behavior, in contrast to the nocturnal
pattern exhibited by wild rabbits (Jilge, 1991).
E.
Reproduction
1.
Sexual Maturity
Puberty generally occurs between the ages of 5–7 months in the New Zealand White rabbit.
Smaller breeds typically reach puberty earlier, and larger breeds a bit later. For
example, Polish or Dutch rabbits are usually sexually mature by 4 months of age, while
Flemish or Checkered Giant rabbits reach sexual maturity by 9 to 12 months.
The breeding life of a doe typically lasts approximately 1 to 3 years, although some
remain productive for up to 5 or 6 years. In later years, litter sizes usually diminish.
In comparison, most bucks will remain reproductively useful for an average of 5 to
6 years.
Because does often will engage in reproductive behavior before being able to ovulate,
it is advisable not to breed does until they are fully grown.
2.
Reproductive Behavior
Does do not have a distinct estrous cycle, but rather demonstrate a rhythm with respect
to receptivity to the buck. Receptivity is punctuated by periods (1–2 days every 4–17
days) of anestrus and seasonal variations in reproductive performance (Hafez, 1970).
During periods of receptivity, the vulva of the doe usually becomes swollen, moist,
and dark pink or red.
Ovulation is induced and occurs approximately 10 to 13 hr after copulation. Interestingly,
up to 25% of does fail to ovulate following copulation. Ovulation can also be induced
by administration of luteinizing hormone (Kennelly and Foote, 1965), human chorionic
gonadotropin (Williams et al., 1991), or gonadotropic releasing hormone (Foote and
Simkin, 1993).
Receptivity of the doe is usually signaled by vulvar changes as described above, restlessness,
and rubbing of the chin on the hutch or cage. Vaginal cytology is generally not useful
for determination of estrus or receptivity in the rabbit.
Typically, the doe is brought to the buck's cage for breeding, since the doe can be
very territorial and may attack the male in her own quarters. A period of 15 to 20
min is usually sufficient to determine compatibility of the doe and buck. If receptive,
the doe will lie in the mating position and raise her hindquarters to allow copulation.
If fighting or lack of breeding is observed, the doe may be tried with another buck.
A single buck is usually sufficient to service 10 to 15 does.
Does may be bred immediately after kindling; however, most breeders delay until after
the kits have been weaned. Success at postpartum breeding varies, but one can produce
a large number of kits in a relatively short time period by foster nursing the young
and rebreeding the doe immediately. While conventional breeding, nursing, and weaning
schedules allow for only 4 litters per year, early postpartum breeding allows for
up to 11 litters per year.
3.
Pregnancy and Gestation
Pregnancy can often be confirmed as early as day 14 of gestation by palpation of the
fetuses within the uterus. Radiographic procedures permit pregnancy determination
as early as day 11. Conception rates have been observed to have an inverse relationship
with ambient temperature but not light cycle. Gestation in rabbits usually lasts for
30 to 33 days. Does beyond 2 to 3 weeks of gestation will usually refuse a buck.
Does begin hair pulling and nest building during the last 3 to 4 days of gestation.
A nesting box with shredded paper or other soft material such as straw should be provided
to the doe several days prior to the expected kindling (parturition) date. The doe
will usually line the box with her own hair. The nesting box should not be placed
in the corner of the cage where the individual doe has been observed to urinate.
4.
Pseudopregnancy
Pseudopregnancy is common in rabbits and can follow a variety of stimuli, including
mounting by other does, sterile matings by bucks, administration of luteinizing hormone,
or the presence of bucks nearby. In such circumstances, ovulation is followed by a
persistent corpus luteum that lasts 15 to 17 days. The corpus luteum or corpora lutea
secrete progesterone during this time, causing the uterus and mammae to enlarge. The
doe may have the appearance of a normally pregnant rabbit. Toward the end of pseudopregnancy,
many does will begin to pull hair as part of ritual nest-building behavior.
5.
Parturition
The process of parturition is referred to as “kindling” as it relates to rabbits.
Kindling normally occurs during the early morning hours and takes approximately 30
to 60 min. Impending kindling is often signaled by nest building and decreased food
consumption during the preceding 2 to 3 days. Both anterior and breech presentations
are normal in the rabbit. Fetuses retained beyond 35 days generally die and may harm
future reproductive ability of the doe if not expelled.
The average number of kits born is 7 to 9 per litter, although smaller litters and
litters up to 10 kits are not uncommon. Litter size is influenced by breed, parity,
nutritional status, and environmental factors. Polish rabbits usually have fewer than
4 kits per litter; Dutch or Flemish, 4 to 5; and New Zealand White, 8 to 10.
After the young have been cleaned following parturition, the doe typically consumes
the placenta. Cannibalism of the young by the doe sometimes occurs and may be related
to environmental or hereditary factors or due to environmental stressors.
6.
Lactation
Does usually have either four or five pairs of nipples, while bucks have none. During
the last week of pregnancy, marked development of the mammary gland occurs. The doe
normally nurses the kits once daily for several minutes, usually in the early morning
or in the evening, regardless of how many kits are present or how many times they
attempt to suckle. Milk yield is normally between 160 and 220 gm/day. Maximum output
occurs at 2 weeks following kindling and then declines during the fourth week. Rabbit
milk contains approximately 12.5% protein, 13% fat, 2% lactose, and 2.5% minerals.
Nursing may last 5 to 10 weeks. Kits may begin consuming solid food by 3 weeks of
age, with weaning generally occurring by 5 to 8 weeks of age.
F.
Management and Husbandry
1.
Housing
The facilities present in most modern research animal facilities would be suitable
for housing rabbits. General construction should include adequate heating, ventilation,
and air conditioning to house rabbits at appropriate temperature and humidity. In
addition, lighting should be adequate to allow easy visualization of the rabbits.
Surfaces, such as the floors, walls, and ceilings, should be easily sanitizable (Suckow
and Douglas, 1997).
Rabbit cages should provide a safe environment with easy access to food and water.
Adults can be caged individually or in compatible groups and should have sufficient
floor space to lie down and stretch out. In the United States, minimum cage sizes
are determined by the Animal Welfare Act (AWA) and the “Guide for the Care and Use
of Laboratory Animals” (1996). In both cases, sizes vary with the weight of the animal.
Currently, the AWA regulations and the “Guide” require 3.0 ft2 of floor space and
14 inches of cage height for rabbits weighing 2–4 kg.
Cages should be constructed of durable materials that will resist corrosion and harsh
detergents and disinfectants used in cleaning. Consequently, in the research environment,
rabbit cages are most often constructed of stainless steel or plastics. Rabbits are
usually housed in cages with mesh or slatted floors to permit urine and feces to drop
through into a catch pan. Mesh floors with catch pans do not prevent rabbits from
engaging in the normal practice of coprophagy.
Rabbits will play with objects placed in their cages. Huls et al. (1991) noted that
rabbits would use wooden sticks, wooden rings, and brass wire balls as toys. Rabbits
can also be housed in compatible pairs or groups, but one should take the possibility
of aggression and pseudopregnancy into consideration before choosing this housing
modality.
2.
Environment
Rabbits require cooler room temperatures than most other common species of laboratory
animals. The “Guide” recommends that temperatures in rabbit rooms be maintained between
61° and 72°F.
No specific illumination requirements for rabbits have been described. It is common
practice to provide rabbits with 12–14 hr of light in the light-dark cycle. In breeding
colonies, females should be provided with 14–16 hr of light.
Ammonia production in rabbit rooms can be a signficant problem; therefore, rabbit
rooms should be ventilated at 10–15 air changes per hr (“Guide,” 1996). It is also
important to change excreta pans often to prevent the buildup of ammonia.
Rabbits are easily startled by sudden, loud noises. For this reason, they should not
be housed near noisy species such as dogs or monkeys, nor should they be housed near
noise-generating operations such as the cage-wash area.
3.
Sanitation
Catch pans should be cleaned as often as necessary to prevent the formation of ammonia.
Cages are generally sanitized on at least a weekly basis.
Rabbit urine contains large amounts of protein and minerals, and often forms deposits
on cages and catch pans. It is common practice to soak equipment having urine deposits
in acid washes to remove the scale before washing.
III.
DISEASES
A.
Bacterial Diseases
1.
Pasteurellosis
Pasteurellosis is a common disease of laboratory rabbits and is caused by Pasteurella
multocida. It is a gram-negative, nonmotile, coccobacillus. Five capsular (A, B, D,
E, and F) and 17 somatic serotypes are currently recognized. Rabbit isolates are most
often capsular types A or D and somatic types 3 or 12 (DiGiacomo et al., 1991). Many
isolates, however, are nontypable (Manning et al., 1989).
Pasteurella multocida causes a variety of clinical syndromes in rabbits. The clinical
presentation can include one or more of the following: rhinitis, sinusitis, pneumonia,
otitis media, otitis interna, conjunctivitis, abscess formation, genital infection,
and septicemia (DeLong and Manning, 1994).
Rhinitis with or without sinusitis is the most common clinical manifestation of pasteurellosis
in rabbits. It is commonly called “snuffles.” In outdoor colonies, the incidence may
be as high as 60%, and the disease is most common in the spring and fall (DiGiacomo
et al., 1983). Rabbits present with a serous to mucopurulent nasal discharge, sneezing,
coughing, and exudate on the fur of the forepaws. Infected rabbits may be clinically
asymptomatic even though the organism is still present in the nasal passages. Rabbits
with rhinitis often develop an associated conjunctivitis. Clinical signs include mucopurulent
ocular exudate, chemosis, conjunctival reddening, swollen eyelids, epiphora, and hair
loss around the eyes (DeLong and Manning, 1994).
Pneumonia is also a common clinical condition in affected rabbits (DeLong and Manning,
1994). Both acute and chronic pneumonia may occur. Chronic pneumonia is often asymptomatic
(DeLong and Manning, 1994; Flatt and Dungworth, 1971). In the research setting, animals
often exhibit few clinical signs because the affected animal's respiratory demands
in the cage are minimal. Affected animals may exhibit anorexia, depression, dyspnea,
moist rales, and death.
Pasteurella multocida can cause otitis media in rabbits, a clinically silent condition
that may progress to otitis interna with torticollis (Fig. 4
) (DeLong and Manning, 1994). Subcutaneous and visceral Pasteurella abscesses can
also be clinically silent for long periods. Subcutaneous abscesses often rupture spontaneously
to the outside.
Fig. 4
Head tilt (torticollis) in a rabbit with otitis interna related to infection with
Pasteurella multocida. Torticollis is often accompanied by circling in the direction
of the affected vestibular apparatus.
Rabbits that develop Pasteurella septicemia generally die acutely without any clinical
signs.
Female rabbits with acute genital tract infections may present with a serous, mucous,
or mucopurulent vaginal discharge. Chronic genital tract infections in the female
are often asymptomatic or may manifest as decreased fertility or abortion. Male rabbits
may develop orchitis or epididymitis, exhibiting decreased fertility and enlarged,
firm testicles.
Rabbits become colonized with P. multocida early in life by the oral or respiratory
routes. Direct contact is the most effective means of transmission, but aerosol transmission
can also occur (DeLong and Manning, 1994). Veneral transmission occurs, but less commonly.
Young rabbits generally become infected around the time of weaning. This probably
corresponds with the decline in maternal antibody that occurs at this time (Glass
and Beasley, 1989). The pharynx may be the first site colonized by P. multocida, with
later spread to the nares and other organs (DeLong and Manning, 1994). Infection rates
as high as 90% have been reported, and the rate of infection in colonies decreases
with age (DeLong and Manning, 1994).
Changes in temperature, experimental manipulation, pregnancy, and concurrent disease
are frequently associated with the development of clinical signs.
The specific pathologic findings will vary with the site of infection, but the underlying
host response is characterized by acute or chronic suppurative inflammation with the
infiltration of large numbers of neutrophils.
Rhinitis and sinusitis are accompanied by a mucopurulent nasal exudate. Neutrophil
infiltration of the tissues is extensive. The nasal passages are edematous, inflamed,
and congested, and there may be mucosal ulcerations. The turbinate bones may atrophy
(DiGiacomo et al., 1989; Chrisp and Foged, 1991). Purulent conjunctivitis may be present.
Pneumonia is primarily cranioventral in distribution. The lungs can exhibit consolidation,
atelectasis, and abscess formation. A purulent to fibrinopurulent exudate is evident,
and there may be areas of hemorrhage and necrosis. In some rabbits, fibrinopurulent
pleuritis and pericarditis are prominent features (Glavits and Magyar, 1990). This
is probably due to elaboration of a heat-labile toxin in some strains of the bacteria
(Chrisp and Foged, 1991). Acute hepatic necrosis and splenic lymphoid atrophy are
also seen in association with the pleuritis and pneumonia induced by toxigenic strains.
Otitis media is characterized by a suppurative exudate with goblet cell proliferation
and lymphocytic and plasma cell infiltration.
In female rabbits with genital tract infections, the uterus may be enlarged and dilated.
In the early stages of infection, the exudate is watery; later it thickens and is
cream-colored. The exudate contains numerous neutrophils. Focal endometrial ulceration
can be found (Johnson and Wolf, 1993). In the male, the testes are enlarged and may
contain abscesses.
Systemic and visceral abscesses are characterized by a necrotic center, an infiltrate
made up of polymorphonuclear neutrophils, and a fibrous capsule.
Septicemia may only present as congestion and petechial hemorrhages in many organs.
Early in the infectious process,
P. multocida
organisms likely colonize the nasopharyngeal mucosa of rabbits. Studies conducted
in vitro
have shown that
P. multocida
is an adhesive organism and has fimbriae (pili) (Glorioso
et al.,
1982; Bonilla-Ruz and Garcia-Delgado, 1993). The factors that lead to subsequent spread
to other organs are unknown.
A variety of bacterial virulence factors likely play a role in protecting the organism
from host defenses. These include resistance to phagocytosis by polymorphonuclear
neutrophils, resistance to killing by serum and complement, toxin production, and
endotoxin production.
Culture of the organism is the most definitive means for diagnosis of pasteurellosis.
Several serologic assays have also been described, including enzyme-linked immunosorbent
assays (ELISA), indirect hemagglutination, and a gel diffusion precipitin test (Lukas
et al., 1987; Zaoutis et al., 1991; Zimmerman et al., 1992; Kawamoto et al., 1994;
Peterson, et al., 1997).
A wide variety of bacterial extracts have been utilized to stimulate a humoral immune
response in rabbits and other species. Presumably, the intent has been to stimulate
opsonizing and/or bactericidal antibodies, but the mechanism(s) by which these vaccines
stimulate protective immunity is (are) not well understood. In general, the host responds
to a component vaccine effective against the homologous organism. In only a few preparations
has protection against heterologous strains been demonstrated. Several antigens that
provide partial protection have recently been described (Lu et al., 1988, 1991; Zimmerman
et al., 1992; Ruffle and Alder, 1996). However, there is no commercial vaccine effective
against
P. multocida
in rabbits.
The control of pasteurellosis in research facilities is most easily accomplished through
the use of
Pasteurella-free
rabbits from commercial vendors. Such animals can be maintained free of the disease
by isolation away from infected animals. Personnel and management procedures should
be established to prevent the introduction of the organism into a
Pasteurella-free
colony.
Minimally, rabbits suspected of harboring
P. multocida
should be isolated from other rabbits and species. Personnel practices such as frequent
changing of lab coats and entering
Pasteurella-free
rabbit rooms only if individuals have had no other contact with other groups on that
day should be undertaken. In addition, the use of laminar flow (outflow) housing and
more rigorous personnel procedures (mask, gloves, gowns, etc.) can be used if the
situation calls for a greater degree of containment.
Development of
Pasteurella-free
rabbit colonies has been accomplished through culture and culling of animals shown
to harbor the organism (Griffin, 1952). Once microbiologically negative animals have
been selected for the colony, that group should be maintained in isolation from other
animals.
Suckow et al. (1996) were able to derive
Pasteurella-free
rabbits by treating pregnant does with enrofloxacin past kindling. Although the
P. multocida
could still be cultured from does, all kits from enrofloxacin-treated does were free
of
P. multocida.
Clinical signs of rhinitis can often be eliminated by treatment with antibiotics.
However, antibiotic therapy will generally not eliminate the organism from the nasal
passages. More serious forms of the disease should be treated with antibiotics after
culture and sensitivity testing of the organism.
Procaine penicillin G (40,000 U/kg IM SID) is very effective against
P. multocida
but should be used with caution to prevent development of clostridial enterotoxemia.
Enrofloxacin at a dosage of 5 mg/kg body weight administered in the drinking water
has been reported as effective (Okerman et al., 1990). Parenteral enrofloxacin (5
mg/kg IM every 12 hr for 14 days) was also shown to be effective, and some rabbits
became culture-negative for
P. multocida
by the end of the course of treatment (Broome and Brooks, 1991).
Tilmicosin (25 mg/kg SC) was shown to be an effective treatment for pasteurellosis
in New Zealand White rabbits (McKay et al., 1996).
The most common research complication associated with pasteurellosis is infection
of injection sites of rabbits immunized for production of polyclonal antisera. Death
of rabbits from
Pasteurella
septicemia can also occur. Vascular cell adhesion molecule-1 (VCAM-1) is expressed
by endothelial cells during inflammation. It has been shown that rabbits infected
with
P. multocida,
compared with uninfected control animals, had increased VCAM-1-positive aortic endothelial
cells (Richardson et al., 1997).
2.
Tyzzer's Disease
The etiologic agent of Tyzzer's disease is
Clostridium piliforme,
a gram-negative, bacillus-shaped, spore-forming bacterium. The disease occurs in many
animal species in addition to rabbits. C.
piliforme
is an obligate intracellular pathogen. The organism cannot be grown in artificial
media and must be cultured in embryonated eggs or tissue culture (Fries, 1977).
The disease occurs most often in young animals, particularly around the age of weaning.
Affected rabbits exhibit profuse, watery diarrhea, anorexia, dehydration, lethargy,
and staining of the hindquarters with feces. Rabbits often die 1–2 days after exhibiting
clinical signs. In acute outbreaks, mortality may be as high as 90% (DeLong and Manning,
1994). Some animals may go on to develop a chronic infection characterized by weight
loss and wasting.
The organism is most likely transmitted via the fecal-oral route through ingestion
of spores. Outbreaks occur most often in 6- to 12-week-old rabbits, but all age groups
are susceptible (DeLong and Manning, 1994). In naive populations, the disease presents
as an epizootic with high morbidity and mortality. In enzootically infected colonies,
many animals may be subclinically infected, but only small numbers may demonstrate
clinical signs (Fries, 1979). Stress may be important in precipitating disease in
subclinically infected rabbits.
Gross necropsy findings include hemorrhages on the serosal surface of the cecum; a
thickened, edematous bowel wall; and foci of necrosis in the mucosa. The ileum and
colon may also be affected. The liver typically has numerous pinpoint white foci throughout
the parenchyma. Similar foci may be present in the myocardium.
Histologically, the cecal lesions consist of subserosal hemorrhages, edema, and mucosal
necrosis that may extend into deeper layers of the cecal wall. The hepatic foci noted
at gross examination correspond to foci of necrosis surrounded by polymorphonuclear
neutrophils.
Multifocal necrotic myocarditis may be seen as well. Tangled masses of rod-shaped
bacteria can be found in the periphery of the lesions using special stains such as
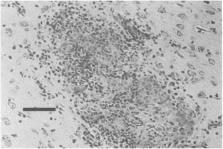
the Warthin-Starry silver stain or Giemsa stain (Fig. 5
) (DeLong and Manning, 1994).
Fig. 5
Focal area of necrosis in a rabbit liver due to Tyzzer's disease. Note the thin Clostridium
piliforme organisms (arrows) within the lesion. Magnification: 400×, Warthin-Starry
silver stain. Bar: 1500 μm.
Clostridium piliforme spores are shed in the feces of infected animals. The disease
is transmitted to susceptible animals by the ingestion of spores contaminating the
environment. Initially, the organism infects the intestinal tract; there is subsequent
systemic spread to other organs, such as the liver and heart. Stress may play an important
role in the development of clinical disease (DeLong and Manning, 1994).
Diagnosis of Tyzzer's disease can be made by demonstrating characteristic intracellular
bacteria in tissue sections stained with the Warthin-Starry silver stain or Giemsa
stain (DeLong and Manning, 1994). Serologic assays are also utilized to diagnose Tyzzer's
disease. Both indirect immunoflourescence and enzyme-linked immunosorbent assays (ELISA)
can be used to detect antibodies to C. piliforme (Fries, 1977; Waggie et al., 1987).
Proper husbandry managment plays an important role in the prevention of Tyzzer's disease.
Sound husbandry practices that maintain high levels of sanitation and minimize stress
should help to reduce the appearance of clinical disease. Within the animal research
setting, only vendors whose animals are known to be free of the disease should be
used. Methods used to screen for latent carriers, such as serum antibody titers or
exposure of gerbils to rabbit feces, might be utilized. Commercial vaccines against
Tyzzer's disease are not available.
Thorough cleansing and disinfection are necessary to decontaminate facilities in which
Tyzzer's disease has occurred. Surfaces should be cleaned with either 1% peracetic
acid or 0.3% sodium hypochlorite (Ganaway, 1980). Spores can withstand repeated freezing
and thawing but are rendered noninfectious after 30 min at 80°C.
Clinical experience indicates that antibiotics, specifically tetracycline or oxytetracyline,
may have some value in controlling epizootics of Tyzzer's disease. However, others
believe that antibiotic treatment is ineffective (DeLong and Manning, 1994).
The principal research complication associated with Tyzzer's disease is death of affected
rabbits. Alterations in serum enzymes as a result of liver damage could also occur.
3.
Enterotoxemia
The primary causative agent of enterotoxemia in rabbits is
Clostridium spiroforme
(DeLong and Manning, 1994). Involvement of other
Clostridium
species has also been reported. Most recently, C.
difficile
was isolated from rabbits (Perkins et al., 1993, 1995).
Clostridium perfringens
type A, and C.
welchii
type A have also been reported as rabbit pathogens.
Most often, affected animals die acutely without clinical signs. Watery brown diarrhea
and staining of the perineal region may be seen. Affected animals may exhibit anorexia,
dehydration, polydipsia, depression, pyrexia or hypothermia, bloat, and grinding of
teeth.
Enterotoxemia can affect rabbits of all ages but is seen primarily in weanling rabbits.
Both isolated cases and epizootics can occur in colonies. In older rabbits, disruption
of the normal gut flora can lead to the development of enterotoxemia.
The cecum may be distended with excessive gas and dark brown fluid, and there may
be serosal paintbrush hemorrhages. There may be mucosal hemorrhages and ulcers in
the cecum. The colon may contain mucus or gas and dark brown fluid, and may feature
an extension of the serosal hemorrhages. The ileum may also be involved. The acute
inflammatory exudate and pseudomembrane formation characteristic of C.
difficile
infections in humans have not been reported in rabbits.
Young rabbits likely develop the disease because of the change in gut flora associated
with weaning. However, several reports of clostridial disease in adult rabbits have
been recorded. These cases may have been associated with other factors that permitted
proliferation of clostridial organisms, such as antibiotic administration, coinfections
with other bacteria, or other stressors (DeLong and Manning, 1994). Cases of primary
clostridial infection with no obvious predisposing factors have also been reported
(Perkins et al., 1995).
Isolation of the causative organism is required to definitively diagnose enterotoxemia.
Procedures for culturing C.
spiroforme
have been described (Borriello and Carmen, 1983). Holmes et al. (1988) recommended
centrifugation of the intestinal contents at 20,000
g
for 15 min, followed by culture of the supernatant-pellet interface.
Clostridium spiroforme
has a characteristic helically coiled, semicircular appearance in fecal smears or
after growth
in vitro.
For a definitive diagnosis, the supernatant from centrifuged cecal contents can be
analyzed for presence of the iota toxin (coupled with bacterial isolation). Both
in vivo
and
in vitro
assays have been described (Patton et al., 1978; Yonushonis et al., 1987; Perkins
et al., 1993, 1995).
Control of enterotoxemia in rabbits should focus on preventing disruption of gastrointestinal
flora through utilization of proper husbandry and veterinary practices. There are
no vaccines available to prevent enterotoxemia in rabbits. Around the time of weaning,
rabbits should not be overfed and should be provided with sufficient dietary fiber.
Abrupt changes in feed should be avoided. Copper sulfate has been advocated as a feed
additive to reduce toxin production by
Clostridium
(DeLong and Manning, 1994). Antibiotics should be used judiciously in rabbits as they
may precipitate enterotoxemia. Parenteral administration is preferred over oral administration.
Treatment for enterotoxemia should include supportive fluid therapy. Although antibiotics
are often recommended, there is little evidence that they are of value in enterotoxemia
(Carman and Wilkins, 1991). Oral cholestyramine (an ion exchange resin) has been proposed
for treatment because it binds bacterial toxins (Lipman et al., 1992).
The principal research complication associated with enterotoxemia is the death of
affected rabbits.
4.
Colibacillosis
In earlier literature, the role of
Escherichia coli
as a causative agent of diarrhea in rabbits was unclear because
E. coli
often proliferates when rabbits develop diarrhea for any reason. Other studies have
demonstrated that certain strains of
E. coli
are capable of causing disease in rabbits.
Escherichia coli
strain RDEC-1 (rabbit diarrhea
E. coli) has satisfied Koch's postulates (Cantey and Blake, 1977). RDEC-1 is now serotyped
as O15:H, one of the more virulent strains that affects weanling rabbits. Strains
(many of which are in serogroup O103) expressing the
eae
gene are most common and are particularly pathogenic in rabbits (Blanco et al., 1996).
This gene encodes intimin, an outer membrane protein required for development of attaching
and effacing lesions (Agin et al., 1996). Also of importance are serotypes O109:H2,
O103:H2, O15:H, O128, and O132 (DeLong and Manning, 1994).
Colibacillosis typically affects 4 to 6-week-old weanlings, but 1- to 2-week-old suckling
rabbits can also be affected. There are three clinical syndromes associated with colibacillosis
depending on the infecting strain of bacteria: neonatal diarrhea with high mortality;
weanling diarrhea with high mortality; and weanling diarrhea with low mortality. Suckling
rabbits typically present with severe yellow diarrhea and high mortality. Weanling
rabbits are more likely to develop profuse, watery diarrhea with dehydration, anorexia,
weight loss, stunted growth, and death if the infecting strain is highly virulent.
Diarrhea can be mild in weanlings infected with strains of low pathogenicity. Neonatal
diarrhea with high mortality is most often associated with serotype O109:H2; weanling
diarrhea with high mortality with serotype O103.H2 or O15:H; and weanling diarrhea
with low mortality with serotypes O123, O128, and O132 (DeLong and Manning, 1994).
The ileal, cecal, and colonic walls may be thickened and edematous, and there may
be mucosal ulcerations. The cecal contents are watery and brown, and there may be
serosal hemorrhages. In neonates, the entire intestinal tract may be affected and
contain yellow-brown feces. Mesenteric lymph nodes may also be enlarged.
Histologically, there is villus atrophy and fusion in the ileum, cecum, and colon.
The epithelium is flattened and disorganized, and there is focal necrosis of the mucosal
epithelium. Neutrophils are present in the lamina propria, and the submucosa is edematous.
Colonies of coliforms may be found on the intestinal surface. Neutrophils and enterocytes
may be present in the intestinal lumen. Attachment of coliforms to the intestinal
mucosal surface and effacement of the epithelial cells lead to a loss of the microvillus
border and secretory diarrhea (Okerman, 1987).
Escherichia coli can be readily cultured from the feces of rabbits with diarrhea.
However, definitive diagnosis requires somatic and flagellar serotyping to correlate
the strain with known enteropathogenic strains.
The use of proper husbandry techniques is important in controlling colibacillosis
in rabbit herds. Good sanitation practices are especially important in stopping the
spread of organisms. Commercial vaccines for colibacillosis are not available for
rabbits.
Treatment for colibacillosis consists primarily of supportive care, such as fluid
and electrolyte replacement. Antibiotics such as chloramphenicol and neomycin have
been used successfully (DeLong and Manning, 1994).
No specific research complications other than mortality have been reported.
5.
Treponematosis
Treponematosis in rabbits is caused by Treponema paraluis cuniculi. It is a gram-negative,
spiral-shaped rod and is closely related to T. pallidum, the causative agent of human
syphilis.
Typical treponemal lesions occur in vulvar or preputial areas (Fig. 6
) and begin with swelling and erythema, often with vesicles or papules. Lesions at
other mucocutaneous junctions can also occur (Fig. 7
). These lesions progress to ulceration, followed by scaling and crusting over the
ulcer. The regional lymph nodes may become enlarged. The lesions are chronic in nature
but may resolve after many weeks.
Fig. 6
Treponematosis. Ulceration with exudation and crusting on the prepuce of a rabbit.
Fig. 7
Treponematosis. Ulceration with exudation and crusting on the nares of a rabbit.
Other names for treponematosis include venereal spirochetosis and rabbit syphilis.
All lagomorphs are susceptible. Clinically apparent disease is uncommon in rabbitries,
while serologic evidence of infection is common. The organism is transmitted between
rabbits during breeding. Rarely, the organism is found in nonbreeding rabbits.
Histopathologic examination of treponemal lesions reveals epidermal hyperkeratosis,
hyperplasia, and acanthosis with ulceration. There may be an exudative crust over
the ulcer. Macrophage and plasma cell infiltration is present. Spirochetes may be
found in the lesion with Warthin-Starry silver stains. The regional lymph nodes may
be hyperplastic.
The organism penetrates the mucous membrane in order to establish infection. Clinical
signs may not appear for 3–6 weeks after exposure, and seroconversion may not occur
until 8–12 weeks after exposure.
Diagnosis of treponematosis can be made by demonstrating spirochetes in the lesions.
The organism has a characteristic spiral morphology. In addition, in wet mounts of
scrapings from lesions examined by dark-field microscopy, the organism demonstrates
corkscrew motility.
Serologic diagnosis can be made using the same assays as are used to diagnose T. pallidum
infection in humans because the two organisms share many antigens. Several tests are
available that vary in sensitivity and specificity. The microhemagglutination
T. pallidum
test is used as a screening test because it is easy to use and sensitive. The venereal
disease research laboratory slide test (VDRL) and the rapid plasma reagin card test
(RPR) are widely available.
New breeding animals should not be introduced into colonies known to be free of treponematosis.
If animals must be introduced, they should be quarantined, tested serologically, and
examined for lesions typical of treponematosis. There are no commercial vaccines to
prevent the disease in rabbits.
Penicillin is effective for treatment of treponematosis. The recommended treatment
consists of three injections of benzathine procaine penicillin (42,000–84,000 IU/kg)
given at 7-day intervals. Lesions should heal within 2 weeks, and RPR and VDRL titers
will decline within several months. Fluorescent treponemal antibody-absorption test
(FTA-ABS) titers may persist for up to a year. If all animals are treated simultaneously,
this regimen can eradicate infection from a colony.
Rabbits infected with
T. cuniculi
are unsuitable for use in research on human trepanematosis because of the close relationship
between the rabbit and human pathogens. Specifically, the presence of shared antigens
would complicate infection and immunologic studies.
6.
Proliferative Enteropathy
Proliferative enteropathy associated with
Lawsonia intracellularis
infection has been described in rabbits (Hotchkiss et al., 1996; Duhamel et al., 1998).
Lawsonia intracellularis
is a curved, gram-negative, obligate intracellular bacterium that plays a key role
in the development of proliferative bowel disease in hamsters (Stills, 1991) and pigs
(McOrist et al., 1993). The organism has been associated with a fatal outbreak of
proliferative enteritis in rhesus monkeys (Klein et al., 1999).
In rabbits, weanlings are most commonly affected. Clinical disease is characterized
by diarrhea, depression, and dehydration, which resolves within 1 to 2 weeks. Disease
rarely results in death. Rabbits can be infected with
L. intracellularis
in the absence of clinical signs (Duhamel et al., 1998). One outbreak with high mortality
was associated with dual infection with enteropathogenic
E. coli
and
L. intracellularis
(Schauer et al., 1998).
Grossly, the most striking finding is thickening and corrugation of the ileum. The
jejunum, cecum, and proximal colon are variably affected as well. The mesenteric lymph
nodes may be enlarged in some animals. In clinically affected rabbits, the cecal contents
appear watery. Microscopically, the intestinal mucosa is thickened; and crowded, elongated,
and sometime branching crypts can be observed (Hotchkiss et al., 1996). Inflammation
is not present in all cases; however, infiltrates consisting of plasma cells and histiocytes
can be observed in some sections. Small intestinal villi are often blunted.
Lawsonia intracellularis
can be found most easily within the cytoplasm of immature crypt epithelial cells of
the ileum. It has not been cultured in cell-free media, but isolates from other species
can be grown in cultured enterocytes (Lawson et al., 1993; Stills, 1991). Diagnosis
can be made by histologic identification of rod-shaped to curved to spiral, silver-staining
bacteria within the apical cytoplasm of crypt enterocytes (Hotchkiss et al., 1996).
Immunohistochemistry can also be used to identify
L. intracellularis
organisms in crypt and villous enterocytes (Schauer et al., 1998). Alternatively,
identification of specific nucleotide sequences by the polymerase chain reaction can
be used to identify the organism in jejunal, ileal, or colonic tissues of infected
rabbits.
Treatment of ill rabbits should be based on symptoms and isolation of sick animals
is advised. Severely diarrheic rabbits should be administered parenteral fluids, and
supplemental heat provided to those that become hypothermic.
B.
Viral diseases
1.
Poxvirus Infections
Myxomatosis, caused by myxoma virus, has a worldwide distribution and is endemic in
the brush rabbit (Sylvilagus bachmani) in the United States. Rabbits of the genus
Oryctolagus are particularly susceptible and often develop a fatal disease characterized
by numerous mucinous skin lesions.
Histopathology shows these “myxomas” to be composed of undifferentiated stellate mesenchymal
cells embedded in a matrix of mucinous material and interspersed with capillaries
and inflammatory cells (DiGiacomo and Maré, 1994). Definitive diagnosis depends on
culture of the virus from infected tissues. Since the disease is spread by fleas and
mosquitoes as well as by direct contact, control measures should include prevention
of contact with arthropods and quarantine of infected rabbits. Vaccines have been
used in Europe with some success.
Rabbits of the genus Sylvilagus develop fibroma-like lesions that may be indistinguishable
from those caused by rabbit fibroma virus. The two diseases have been distinguished
by inoculation of fibroma material into Oryctolagus rabbits. They develop a fatal
disease if the myxoma virus is the etiologic agent, or fibromas if rabbit fibroma
virus is responsible.
Rabbit (Shope) fibroma virus is a poxvirus that is antigenically related to myxoma
virus. Fibromatosis is endemic in wild rabbits; however, an outbreak in commercial
rabbits caused extensive mortality (Joiner et al., 1971). Usually, less virulent strains
cause skin tumors in domestic rabbits (Raflo et al., 1973). The disease is probably
spread by arthropods, although definitive evidence is lacking (DiGiacomo and Maré,
1994). Fibromas are flat, subcutaneous, easily movable tumors; whereas papillomas
arise from the skin, are heavily keratinized, and project outward.
Rabbit pox is a rare disease induced by a poxvirus that has caused outbreaks of fatal
disease in laboratory rabbits in the United States and Holland (DiGiacomo and Maré,
1994). Rabbits with the disease may or may not present with “pox” lesions in the skin.
The animals have a fever and nasal discharge 2 or 3 days after infection. Most rabbits
have eye lesions including blepharitis, conjunctivitis, and keratitis with subsequent
corneal ulcers. Skin lesions, when present, are widespread. They begin as a rash and
progress to papules up to 1 cm in diameter by 5 days postinfection. The lymph nodes
are enlarged, the face is often edematous, and there may be lesions in the oral cavity.
At gross necropsy, there are extensive nodules in many organs, and there is widespread
necrosis. Characteristic cytoplasmic inclusions seen in many poxvirus infections are
rare in this disease. The virus is apparently spread by aerosols and is difficult
to control.
2.
Herpesvirus Infections
Two herpesviruses have been isolated from rabbit kidney cultures. These are Leporid
herpesvirus 1 (Herpesvirus sylvilagus), isolated from cottontail rabbits, and Leporid
herpesvirus 2 (Herpesvirus cuniculi), isolated from domestic rabbits. Neither of these
isolates has been shown to cause naturally occurring disease. Experimentally, Leporid
herpesvirus 1 causes a lymphoproliferative disease in inoculated cottontail rabbits
(DiGiacomo and Maré, 1994). Acute mortality was associated with an unknown herpesvirus
isolated from the kidneys of 4 adult rabbits from two commercial rabbitries (Onderka
et al., 1992). Experimental inoculation of rabbits with the virus reproduced the disease
syndrome. The virus has not been well documented.
3.
Papillomavirus Infections
The cottontail rabbit is the natural host of the cottontail (Shope) papillomavirus,
which causes horny warts primarily on the neck, shoulders, and abdomen. The disease
has a wide geographic distribution with the highest incidence occurring in rabbits
in the Midwest (DiGiacomo and Maré, 1994). However, natural outbreaks in domestic
rabbits have been reported (Hagen, 1966). In these natural outbreaks, papillomas were
more common on the eyelids and ears. A small percentage of papillomas are transformed
into squamous cell carcinoma, indicating that this virus is oncogenic. The virus has
been transmitted experimentally by arthropods. Therefore, arthropod control could
be used as a means to prevent the disease from being transmitted to domestic rabbits.
This virus is used extensively as a model for the study of oncogenic virus biology
and as a model for the induction of protective immunity against papillomaviruses (Salmon
et al., 1997; Sundaram et al., 1998).
Rabbit oral papillomatosis is caused by a different virus than that causing cottontail
rabbit papillomatosis. Naturally occurring lesions have been seen in laboratory rabbits
and appear as small, white, discrete growths on the ventral surface of the tongue.
Microscopic examination shows them to be typical papillomas. Most lesions eventually
regress spontaneously (DiGiacomo and Maré, 1994).
4.
Rotavirus Infections
A number of studies have shown that rotavirus infections in rabbits are common (DiGiacomo
and Maré, 1994). Many colonies of rabbits are serologically positive, and rotavirus
can be isolated readily from rabbit feces. However, attempts to experimentally produce
clinical disease have had variable results. Mild diarrhea is usually seen, but in
some studies there has been significant mortality. It is probable that rotavirus is
only mildly pathogenic in rabbits and may require the presence of other organisms
in order to produce clinical disease. In combined experimental infections with both
rotavirus and Escherichia coli, the inoculation of both organisms led to more serious
clinical signs than when given alone, indicating that rotavirus may have been a more
significant determinant in the manifestation of this disease (Thouless et al., 1996).
These investigators also showed that older rabbits were naturally more resistant to
the combined infection with rotavirus and E. coli. Very young rabbits appear to be
protected from rotavirus infection by passive immunity, when present, but are quite
susceptible when there is none (Schoeb et al., 1986). Rabbits of weaning age seem
to be the most susceptible. This is also the time when they are most likely to be
subjected to diet changes that may contribute to a change in microbial flora.
5.
Coronavirus Infections
Pleural effusion disease/infectious cardiomyopathy was diagnosed in rabbits inoculated
with Treponema pallidum–infected stocks of testicular tissue. Because these treponemes
could not be grown in vitro, the organism was propagated by passage in rabbits. The
stocks were contaminated with a Coronavirus, although it is not known whether this
virus originated from rabbits or was a virus of human origin that had adapted to rabbits.
With continued passage, the virus became more virulent, and significant mortality
ensued. Evidence indicated that it was not transmitted by direct contact. Rabbits
died due to congestive heart failure, and microscopic examination showed there was
widespread necrosis of the heart muscle. It has been suggested that infection with
this virus might be a model for the study of virus-induced cardiomyopathy (DiGiacomo
and Maré, 1994).
Rabbit enteric Coronavirus has been isolated from tissue cultures from rabbits (LaPierre
et al., 1980) and has been associated with one naturally occurring outbreak of diarrhea
in a barrier-maintained breeding colony (Eaton, 1984). These rabbits developed severe
diarrhea, and most died within 48 hr of onset of clinical signs. Attempts to reproduce
the disease led to watery diarrhea, which lasted a short time; however, none of the
rabbits died. It is quite probable that other microorganisms or unknown environmental
factors contributed to the severity of this outbreak.
6.
Calicivirus Infections
Rabbit hemorrhagic disease (RHD), determined to be caused by a calicivirus, was first
reported in China in 1984 and has since spread to other parts of Asia and Europe.
More recently, the virus escaped from an island near Australia and has since caused
widespread deaths on the mainland (Chasey, 1997). In addition, outbreaks have recently
been reported in the United States and Mexico. The incubation period may be as short
as 1 or 2 days, and sudden death with no previous signs is not uncommon. Clinical
signs may included lethargy, anorexia, and fever. Periportal hepatic necrosis is the
only consistent microscopic lesion, and the animals die due to disseminated intravascular
coagulation with deep venous thromboses. The virus had not been successfully grown
in vitro; however, diagnosis can be confirmed with negative-contrast electron microscopy
of liver tissue. Specific antibodies can be detected by monoclonal antibody ELISA
or by hemagglutination inhibition. The agent resists drying, can be carried on fomites,
and may be transmitted via respiratory and intestinal secretions (Mitro and Krauss,
1993). Any rabbit colonies with this disease should be quarantined and depopulated,
and the environment thoroughly cleansed and disinfected.
Another calicivirus, European brown hare virus, has caused disease in hares in several
countries in Europe (DiGiacomo and Maré, 1994). Animals present with necrotic hepatitis,
hemorrhages in the trachea and lungs, and pulmonary edema. Results of experimental
inoculation of domestic rabbits are mixed, with some investigators reporting a disease
similar to RHD, while others failed to induce disease. The virus is similar to that
of RHD, but not identical. A monoclonal antibody ELISA is available, and control measures
are similar to those for RHD.
7.
Other Viral Infections
Several other viruses have been isolated from rabbit tissues, but have not been shown
to produce disease. These include paramyxoviruses and bunyaviruses. Serologic titers
to to-gaviruses and flaviviruses have also been demonstrated in rabbit antisera (DiGiacomo
and Maré, 1994).
C.
Protozoal Diseases
1.
Hepatic Coccidiosis
Hepatic coccidiosis is caused by the parasite Eimeria stiedae, which has also been
referred to as Monocystis stiedae, Coccidium oviforme, and Coccidium cuniculi (Hofing
and Kraus, 1994). The age of the host strongly affects parasite development and oocyst
production. Four-month-old, coccidia-free rabbits experimentally infected with E.
stiedae produced fewer oocysts than similarly infected 2-month-old rabbits (Gomez-Bautista
et al., 1987).
The clinical disease has a wide range of manifestations. Mild infections often result
in no apparent disease. Most clinical signs are the result of interruption of normal
hepatic function and blockage of the bile ducts. Diarrhea can occur at the terminal
stages of the disease (Hofing and Kraus, 1994). Enlargement of the liver (hepatomegaly)
is common. The liver normally is approximately 3.7% of the body weight, but rabbits
with severe hepatic coccidiosis may have livers that contribute to greater than 20%
of the body weight (Lund, 1954a). Serum bilirubin levels can rise to 305 mg/dl, increasing
as soon as day 6 of infection and increasing through days 20–24 before moderating
(Rose, 1959). Decreased growth rates and weight loss are common. Joyner et al. (1987)
demonstrated that infested rabbits begin to lose weight within 15 days.
Eimeria stiedae is found worldwide, although rabbits bred for use in research are
commonly free of the parasite. Transmission occurs by the fecal-oral route, as for
other coccidia. The organism has also been experimentally transmitted by intravenous,
intraperitoneal, and intramuscular administration of oocysts (Pellérdy, 1969).
Necropsy often shows the liver to be enlarged and discolored, with multifocal yellowish
white lesions of varying size. Exudate in the biliary tree is common, along with dilatation
of bile ducts. Microscopically, papillomatous hyperplasia of the ducts along with
multiple life-cycle stages of the organism in the biliary epithelium can be seen.
Smetana (1933) demonstrated that infection of the entire liver occurred following
ligation of the right bile duct and inoculation of E. stiedae oocysts. The study also
showed that infection occurred earliest within the small intrahepatic ducts, leading
to the theory that infection occurred via blood or lymph. The precise life cycle is
still undetermined, although a number of studies have examined it (Rose, 1959; Horton,
1967; Owen, 1970). Sporozoites have been demonstrated in the lymph nodes following
experimental inoculation (Rose, 1959; Horton, 1967).
Diagnosis can be made by examination of fecal material, by either flotation or concentration
methods. Oocysts can also be detected within the gallbladder exudate (Hofing and Kraus,
1994). Alternatively, oocysts can sometimes be observed by microscopic examination
of impression smears of the cut surface of the liver.
Control of the infection until development of natural immunity is one strategy to
minimize the severity of disease. Davies et al. (1963) demonstrated that immunity
occurs following a light infection with E. stiedae. In the rabbit, immunity to Eimeria
may be lifelong (Pellérdy, 1965; Niilo, 1967). Prevention of hepatic coccidiosis with
sulfaquinozaline in the feed (250 ppm) was shown to prevention infection in the face
of experimental challenge with 100,000 sporulated oocysts (Joyner et al., 1987). Sulfonamides
have been shown effective against Eimeria spp. (Jankiewicz, 1945; Horton-Smith, 1947;
Lund, 1954b; Hagen, 1958; Tsunoda et al., 1968). Development of the organism was arrested
by treatment with 0.02% sulfamerazine sodium administered continually in the drinking
water (Peterson, 1950). Thorough sanitation of potentially contaminated surfaces is
critical to control of coccidiosis.
Potential research complications arising from hepatic coccidiosis are considerable.
The resulting liver damage and decreased weight gains can complicate both the supply
of rabbits for research as well as adversely affect the research protocol.
2.
Intestinal Coccidiosis
There are at least eight different pathogenic species of intestinal coccidia in rabbits,
including E. intestinalis, E. flavescens, E. irresidua, E. magna, E. media, E. piriformis,
E. neoleporis, and E. perforans (Varga, 1982). All of these coccidia are presented
here as a group rather than as individual species of intestinal coccidia.
Although intestinal coccidiosis may be subclinical, symptoms can range from mild to
severe and can result in death of the animal. Postweanling rabbits are the most likely
to experience mortality related to intestinal coccidiosis. Clinical signs also depend
on the species of coccidia that are present. Severe diarrhea, weight loss, or mild
reduction in growth rate are all possibilities. Death is usually associated with severe
dehydration subsequent to diarrhea (Frenkel, 1971).
Intestinal coccidiosis is a common rabbit disease worldwide (Varga, 1982). Transmission
is by the fecal-oral route, through ingestion of sporocysts. Unsporulated oocysts
are passed in the feces and are not infective. Such oocysts will, however, sporulate
to an infective stage within 3 days after shedding; thus, it is important that sanitation
be frequent enough to remove infective stages from the environment. The oocyst burden
of feces can be enormous. Gallazzi (1977) demonstrated that an asymptomatic carrier
of intestinal coccidia had 408,000 oocysts/gm of feces and that a rabbit with diarrhea
could have in excess of 700,000 oocysts/gm of feces. Environmental contamination with
oocysts can be a problem when large numbers of oocysts are being excreted.
Lesions are apparent in the small and large intestines. Necrotic areas of the intestinal
wall appear as white foci (Pakes, 1974; Pakes and Gerrity, 1994). The location and
extent of the lesions depend on the species of coccidia.
The life cycles of Eimeria spp. are similar to those of other coccidia. Schizogony,
gametogony, and sporogony are the three phases of this life cycle. Other sources can
be consulted for greater detail on the life cycle of this protozoan (Rutherford, 1943;
Davies et al., 1963; Pellérdy, 1965).
Diagnosis of intestinal coccidiosis can be made through identification of the oocysts
in the feces (Pakes, 1974; Pakes and Gerrity, 1994). Using polymerase chain reaction
(PCR) technology, a diagnostic test has been developed to detect Eimeria spp. in the
feces. The test can be used to detect as few as 30 sporulated oocysts in rabbit feces
(Cere et al., 1996). A 5S ribosomal RNA species-specific probe exists for E. tenella,
a common parasite of poultry; however, the test is also useful for differentiating
E. tenella from other Eimeria species (Stucki et al., 1993).
Since intestinal coccidiosis is most common in postweanling rabbits, prevention of
the disease should focus on the preweaning period. An oral vaccination has been developed
and consists of a nonpathogenic strain of E. magna. This vaccine is sprayed into the
nest box when rabbits are 25 days of age. The preweanling rabbits develop immunity
subsequent to infection with the nonpathogenic strain and are then resistant to wild-type
strains of E. magna at 35 days of age (Drouet-Viard et al., 1997). Prevention and
control of infection can be accomplished by providing 0.02% sulfamerazine or 0.05%
sulfaquinoxaline in the drinking water (Kraus et al., 1984). A combination of sulfaquinoxaline,
strict sanitation, and elimination of infected animals has been shown to eliminate
intestinal coccidiosis from a rabbit breeding colony (Pakes and Gerrity, 1994). As
for hepatic coccidiosis, sulfaquinoxaline provided in the feed (250 ppm) is an effective
treatment.
3.
Cryptosporidiosis
The protozoan organism Cryptosporidium cuniculus has been found in the intestinal
tract of the rabbit (Inman and Takeuchi, 1979; Rehg et al., 1979). The name is based
on the assumption of host specificity (Pakes and Gerrity, 1994), although C. parvum
has been shown to have a wide host range across mammalian species, including humans
(Current and Garcia, 1991). Transmission is likely be ingestion of thick-walled sporulated
oocysts. Clinical signs related to cryptosporidiosis have not been well described
in the rabbit, although one report describes small intestinal dilatation observed
during surgery in a rabbit that did not have diarrhea (Inman and Takeuchi, 1979).
Histopathology of the small intestine of the reported rabbit was characterized by
shortened, blunted villi and mild edema of the lamina propria. The lacteals of the
ileum were also dilated. Interestingly, inflammatory response was observed.
4.
Encephalitozoonosis
The etiologic agent responsible for encephalitozoonosis is Encephalitozoon cuniculi.
This agent is historically known by the name Nosema cuniculi (Pakes and Gerrity, 1994).
The disease was first described in 1922 as an infectious encephalomyelitis causing
motor paralysis in young rabbits (Wright and Craighead, 1922).
Although named for the motor paralysis in the young rabbit, the disease is usually
latent in rabbits. Other clinical signs can include convulsions, tremors, torticollis,
paresis, and coma (Pattison et al., 1971).
Routes of transmission are not known. The organism has been found in the urine of
infected rabbits (Yost, 1958). Transmission has occurred by oral administration of
urine from infected rabbits (Cox et al., 1979). Evidence for vertical transmission
in the rabbit has been reported (Hunt et al., 1972). This case report describes 2
litters of rabbits that were delivered by cesarean section, raised in a germfree environment,
and fed sterile food. At 2 months of age, 2 were sacrificed due to poor weight gain.
At necropsy, typical lesions of encephalitozoonosis were seen.
The kidneys commonly demonstrate lesions. Typically, there are multiple white, pinpoint
areas or gray, indented areas on the renal cortical surface (Kraus et al., 1984).
Microscopically, these areas are characterized by granulomatous inflammation. Interstitial
infiltration of lymphocytes and plasma cells and tubular degeneration may also be
present (Flatt and Jackson, 1970). Granulomatous encephalitis is a characteristic
lesion (Fig. 8
) (Pakes and Gerrity, 1994). Lesions of the spinal cord can also occur (Koller, 1969).
The organisms are often not observed in histologic sections of the lesions. Organisms
may be seen floating free in the tubules of the kidney (Pakes and Gerrity, 1994).
Fig. 8
Granulomatous encephalitis related to infection with Encephalitozoon cuniculi. The
E. cuniculi organisms are rarely seen within such lesions. Magnification: 1000×. Bar:
3750 μm.
Although the pathologic changes associated with the organism have been well described,
there is little known concerning the development of the disease. The organism can
be found in the tissues without an inflammatory response (Pakes and Gerrity, 1994).
It has been postulated that the rupture of cells containing the organism may induce
the granulomatous reaction (Koller, 1969). Encephalitozoonosis is also a newly recognized
disease in immunodeficient humans. It is recommended that such individuals seek medical
counsel prior to handling rabbits. Isolates from humans have been shown to be infectious
for rabbits (Mathis et al., 1997).
Definitive diagnosis can be made using several different methods. Histologic examination
of tissues and observation of the organism is definitive. The Encephalitozoon organism
does not stain well with hematoxylin and eosin, and is better demonstrated using Giemsa
stain, Gram stain, or Goodpasture-carbol fuchsin stain (Pakes, 1974). Many different
serologic tests exist for the organism. The indirect fluorescence antibody technique
has shown good results in screening large colonies of rabbits (Cox and Gallichio,
1978). Other tests include the complement fixation test, an immunoperoxidase test
(Wosu et al., 1977; Gannon, 1978), a microagglutination test (Shadduck and Geroulo,
1979), and an enzyme immunoassay (Cox et al., 1981).
An indirect fluorescence antibody test has been used to identify spores in the urine
and tissues. Advances in diagnostic techniques have been made in human medicine due
to the susceptibility of immunosuppressed patients to this particular infection. Several
PCR tests for diagnosis and species differentiation of encephalitozoonosis have been
developed (Croppo et al., 1998; Franzen et al., 1998; Weiss and Vossbrinck, 1998).
Although these tests have generally not been used for diagnostic purposes in rabbits,
they offer a wide range of diagnostic possibilities in humans. Amplification of the
organism from urine, tissue biopsies, and feces has been described (Weiss and Vossbrinck,
1998).
Prevention and control of the organism in the colony are done by elimination of the
organism from the colony. Because this is a latent disease in rabbits, serologic methods
must be used to identify carriers of the organism. The indirect fluorescence antibody
test has been used successfully to identify infected rabbits (Cox, 1977). The elimination
of infected rabbits must be accompanied by disinfection of the environment. Several
disinfectants have been effective against this organism. Encephalitozoon was killed
by 2% (v/v) Lysol, 10% (v/v) Formalin, and 70% (v/v) ethanol (Shadduck and Polley,
1978).
Successful treatment in the rabbit has not been reported (Pakes and Gerrity, 1994).
Albendazole has been used successfully in human cases of E. intestinalis (Weber et
al., 1994; Molina et al., 1998). This drug may show some promise for treatment in
rabbits; however, the majority of infections in rabbits are asymptomatic.
Encephalitozoonosis is most commonly an asymptomatic disease, which makes it difficult
to determine the effects it may have on research. Granulomatous reactions would obviously
complicate renal physiology and neurologic research. Depression of the IgG response
and an increase in the IgM response to Brucella abortus antigens has been demonstrated
in rabbits infected with Encephalitozoon organisms (Cox, 1977). Natural killer cell
activity is also increased in mice infected with the organism.
D.
Arthropod and Helminth Diseases
1.
Psoroptes cuniculi (Rabbit Ear Mite)
Psoroptes cuniculi is a nonburrowing mite and the causative agent of psoroptic mange,
also called ear mange, ear canker, or otoacariasis. The organism is distributed worldwide.
Lesions occur primarily in the inner surfaces of the external ear. The lesions are
pruritic and can result in scratching, head shaking, pain, and even self-mutilation
(Hofing and Kraus, 1994). A tan, crusty exudate accumulates in the ears over the lesions
and can become quite extensive and thick (Fig. 9
). The skin under the crust is moist and reddened. The ears may become malodorous.
Fig. 9
Crusty exudate from the ear of a rabbit infested with ear mites (Psoroptes cuniculi).
All stages of the mite (egg, larva, protonymph, and adult) occur on the host. Early
in the infestation, mites feed on sloughed skin cells and lipids. As local inflammation
increases, they ingest serum, hemoglobin, and red blood cells (DeLoach and Wright,
1981; Hofing and Kraus, 1994). The entire life cycle is complete in 21 days. Mites
are relatively resistant to drying and temperature and can survive off the host for
7–20 days in a temperature range of 5°–30°C and relative humidity of 20–75%.
Lesions are characterized histologically by chronic inflammation, hypertrophy of the
Malpighian layer, parakeratosis, and epithelial sloughing. An allergic response to
the mites and mite feces and saliva is likely involved (Hofing and Kraus, 1994).
Mites are large enough to be seen with the unaided eye or with an otoscope. Material
scraped from the inner surface of the ear can also be examined using a dissecting
microscope. Mites are oval-shaped with well-developed legs that project beyond the
body margin. Adult males measure 431–547 μm × 322–462 μm, and females measure 403–749
μm × 351–499 μm (Hofing and Kraus, 1994).
Several successful treatments have been reported. Prior to local treatment, the ears
should be cleaned gently to remove accumulated exudate. One treatment involves the
application of 3% rotenone in mineral oil (1:3) every 5 days for 30 days. Ivermectin
is an effective treatment at dosages of 400–440 μg/kg SC or IM (Wright and Riner,
1985; Curtis et al., 1990; McKellar et al., 1992). One or two doses were utilized
for effective treatment. Bowman et al. (1992) reported an efficacy of 99.6% in rabbits
with a single dose of 200 μg by the SC route. It is generally recommended that the
entire group of rabbits be treated at the same time. Heat (40°C) and desiccation (<
20% humidity) will kill parasites that are not on the host (Arlain et al., 1984).
2.
Cheyletiella spp. (C. parasitovorax, C. takahasii, C. ochotonae, C.johnsoni)
Cheyletiella mites are nonburrowing skin mites of rabbits. They are distributed worldwide.
Several closely related species have been reported to occur on rabbits, namely, C.
parasitovorax, C. takahasii, C. ochotonae, and C. johnsoni (Hofing and Kraus, 1994).
The anatomic site most commonly infested is the area over the scapulae. There may
be mild hair loss in the area, and the skin may have a gray-white scale (Fig. 10
) (Cloyd and Moorhead, 1976). Affected rabbits do not scratch, and there is no evidence
of pruritis (Hofing and Kraus, 1994).
Fig. 10
Hair loss and white scaling in a rabbit infested with skin mites (Cheyletiella spp.).
a more typical location for this lesion is on the back in the scapular region.
All stages (egg, larva, pupa, and adult) in the life cycle occur on the host. Mites
remain in association with the keratin layer of the skin and feed on tissue fluid
(Myktowyz, 1957). Transmission is probably by direct contact.
Skin lesions are mild or nonexistent. When present, lesions are characterized by mild
dermatitis, hyperkeratosis, and an inflammatory cell inflitrate (Hofing and Kraus,
1994).
Mites can be isolated by scraping or brushing fur in the affected areas. Samples may
be cleared with 5–10% potassium hydroxide to improve viewing. Mites can be identified
under a dissecting microscope. The female measures 450 × 200 μm, and the male is 320
× 160 μm. Cheyletiella mites have a large, distinctive curved claw on the palpi (Pegg,
1970).
Topical acaricides are often used and are effective at controlling infestation. Alternatively,
ivermectin may be used as described for Psoroptes cuniculi.
Cheyletid mites can cause a transient dermatitis in humans who are in regular contact
with infested animals (Cohen, 1980; Lee, 1991). For this reason, these mites are considered
a zoonotic pathogen.
3.
Sarcoptes scabiei
Sarcoptes scabiei is a burrowing mite and the causative agent of sarcoptic mange.
Mites of the genus Sarcoptes are generally considered to be one species, S. scabiei,
but are often further identified by a variety name corresponding to the host species
(e.g., S. scabiei var. cuniculi). The organisms are commonly referred to as itch or
scab mites. The disease has a worldwide distribution.
Notoedric mites (Notoedres cati) are similar to sarcoptic mites in morphology, life
cycle, and public health significance. Mites burrow and produce an intensely pruritic
dermatitis. The lesions occur primarily on the face, neck, and ears of rabbits.
Affected rabbits will exhibit intense pruritis. There is often hair loss and abrasions
as a result of the scratching. Serous encrustations on the skin and secondary bacterial
infections are common. Lesions are most common on the head (Hofing and Kraus, 1994).
Anemia and leukopenia can also be observed in affected rabbits (Arlain et al., 1988).
All stages of sarcoptic mange mites occur on the host. The females burrow into the
skin to lay eggs. Young larvae can also be found in the skin while older larvae, nymphs,
and males reside on the skin surface. Mites feed on lymph and epithelial cells (Hofing
and Kraus, 1994).
Amyloidosis of the liver and glomerulus has been reported in rabbits with severe infestation
(Arlain et al., 1990).
Because Sarcoptes is a burrowing mite, skin scrapings are necessary to diagnose infestation.
Samples may be cleared with 5–10% potassium hydroxide. Female mites measure 303–450
μm × 250–350 μm. The body shape is round, and the legs are very short.
Ivermectin is effective at eliminating infestation at 100 μg/kg administered subcutaneously.
Sarcoptes can cause a self-limiting dermatitis in humans. Transmission is by direct
contact.
4.
Other Arthropod Parasites
A wide variety of arthropod parasites have been reported in wild rabbits but are extremely
rare in laboratory rabbits. For an extensive listing the reader is referred to other
sources (Hofing and Kraus, 1994).
5.
Oxyuriasis (Pinworm Infestation)
Pinworms are occasionally found in the cecum and colon in laboratory rabbits. Historically,
the rabbit pinworm was identified as Oxyuris ambigua, but this name is synonymous
with the more contemporary name, Passalurus ambiguus (Hofing and Kraus, 1994).
Even when rabbits have heavy oxyurid burdens, clinical signs are not usually apparent
(Erikson, 1944; Soulsby, 1968). One case report describes unsatisfactory breeding
performance and poor condition in a rabbit herd infested with the parasite.
Passalurus ambiguus can readily be found in wild rabbits as well as in domestic and
research rabbits (Hofing and Kraus, 1994). Transmission occurs easily, given that
individual rabbits have been found with over 1000 adult parasites (Hofing and Kraus,
1994) and that embryonated eggs pass out in the feces and are immediately infective
(Taffs, 1976).
Mature pinworms are found in the lumen of the cecum or colon of the rabbit. After
ingestion, the eggs hatch in the small intestine, and the larvae molt. Development
continues, and maturation occurs in the cecum. The prepaient period is between 56
and 64 days (Taffs, 1976).
Several successful treatment strategies for rabbit oxyuriasis have been reported.
Piperazine citrate at 100 mg/100 ml of drinking water for 1 day was successful in
eliminating infestation (Hofing and Kraus, 1994). Fenbendazole mixed in the food for
5 days was effective at several dose levels. At 12.5 ppm, 99% of adult and most immature
pinworms were eliminated. At 25 and 50 ppm, fenbendazole eliminated all immature and
adult pinworms (Duwell and Brech, 1981). One gram of phenothiazine in 50 gm of feed
has also been used. Subcutaneous doses of ivermectin (0.4 mg/kg) were reported to
be ineffective in reducing the burden of
Passalurus
organisms in field populations of snowshoe hares (Lepus americanus) (Sovell and Holmes,
1996).
E.
Mycotic Diseases
Fungal forms are omnipresent in the environment. Evaluations of airborne fungi in
an animal facility showed that counts of viable fungus particles were, in general,
low.
Penicillium
was the most commonly recovered type,
Aspergillus fumigatus
was rarely recovered, and dermatophytes were not recovered. It appeared that bedding
was the principal source of these fungi and that outdoor airborne fungi did not markedly
contribute to the indoor air fungi identified (Burge et al., 1979.)
1.
Superficial Mycoses
Dermatophytosis is synonymous with the more colloquial descriptive term, “ringworm.”
The clinical disease is common among pet rabbits but is seen infrequently in laboratory-bred
and -maintained animals. This is likely the result of the higher standard of husbandry,
especially disinfection, followed by most research facilities. Marginal husbandry
practices, poor nutrition, environmental stressors, overcrowding, excessive heat or
humidity, extremes of age, and pregnancy are all factors that might precipitate clinical
disease. Clinical dermatophytosis most commonly affects the occasional individual,
although epizootic outbreaks have been described (Flatt et al., 1974). Endemic dermatophytosis
that spread to employees and their families has also been described (Szili and Kohalmi,
1981). It should be noted that dermatophytosis is a zoonotic disease, and affected
rabbits should be handled in a manner that will minimize the exposure of personnel
to the pathogen.
The causative agent most commonly identified with clinical dermatophytosis is
Trichophyton mentagrophytes,
with
Microsporum canis
being identified on occasion. In rare instances,
T. rubrum
or
M. gypseum
is isolated (Flatt et al., 1974).
Transmission of the agent occurs through direct contact with affected individuals
or with macroconidia and arthrospores in the environment. Fomites can be a significant
source of infection, particularly objects such as hairbrushes or other equipment that
might be used without proper disinfection between animals. Asymptomatic carriers are
not uncommon, with one study isolating
T. mentagrophytes
from 36% of clinically normal rabbits (Lopez-Martinez et al., 1984)
Clinical disease is characterized by patchy alopecia with crusting, especially on
the head and face. Lesions are often erythematous. The disease may spread to the paws,
ears, and other sites. The lesions are typically pruritic, circular, and 1–2 cm in
diameter, and have a peripheral raised rim of acute inflammation and broken hairs.
Similar to dermatophytosis in other species, the lesion expands radially with central
healing. Hyperkeratosis and acanthosis are characteristic histologic findings, with
acute and chronic inflammatory cells diffusely infiltrating the underlying dermis.
Focal abscesses of the hair follicles within the perimeter of the lesion commonly
occur because of secondary bacterial invasion. Special stains such as periodic acid–Schiff,
Gridley fungus stain or Gomori methenamine–silver stain are required to visualize
mycelia and arthrospores.
Although the lesions described above are characteristic of dermatophytosis, diagnostic
procedures should be performed to definitively differentiate the condition from other
skin diseases such as acaritic mange, fur pulling, moist dermatitis, malnutrition,
spirochetosis, seasonal molting, behavioral vice, and bacterial dermatopathy. Following
a physical examination and determination of the clinical history of the animal, skin
scrapings with mineral oil and 10% potassium hydroxide (KOH) should be performed.
The mineral oil scraping should be examined for ectoparasites. The KOH scraping should
be placed in the KOH for 30–40 min and then gently heated for 10 min prior to examination
for mycelia or arthrospores. In either case, scrapings should be taken from the periphery
of the lesion. Dermatophytosis can also be confirmed by viewing the lesion under a
Wood's lamp. Some isolates of
Microsporum
fluoresce under Wood's lamp illumination. However,
Trichophyton
and some
Microsporum
isolates do not fluoresce, thus a negative result with the Wood's lamp does not rule
out dermatophytosis. Finally, samples of hair plucked from the edge of the lesion
can be cultured for dermatophytes, using special media such as dermatophyte test media
(DTM) or Sabouraud's agar. A positive culture should be followed by confirmation of
fungal forms on a KOH skin scraping preparation or in the hair follicles by histopathology
of a biopsy.
Isolation of rabbits suspected of having an active dermatophyte infection is critical,
since people and other rabbits and animals are at risk if exposed. Affected rabbits
can be treated with griseofulvin (25 mg/kg) by gastric intubation once daily for 14
days. Affected rabbit colonies can be effectively treated with medicated diets containing
0.375 gm of griseofulvin per lb of diet for 14 days (Hagen, 1969). Alternatively,
affected rabbits can be treated with 1% copper sulfate applied as a dip or with a
dilution of a metastabilized chlorous acid-chlorine dioxide compound applied as either
a dip or a spray (Franklin et al., 1991).
2.
Deep and Systemic Mycoses
Systemic and deep mycoses are rare in rabbits. Aspergillosis associated specifically
with Aspergillus flavus or A. fumigatus has been reported sporadically (Flatt et al.,
1974). Pulmonary aspergillosis has been described in otherwise healthy young rabbits
at a rabbitry in Japan (Matsui et al., 1985). Lesions contained hyphae surrounded
by eosinophilic “asteroid bodies.” Isolation of A. fumigatus from the reproductive
tract of an adult female rabbit that aborted at an advanced stage of pregnancy and
the associated placenta has also been reported (Boro et al., 1978).
Pneumocystis carinii is a microorganism present in the lungs of many mammal species.
Although the exact taxonomic classification has been debated, recent studies strongly
suggest that P. carinii is a fungus (Edman et al., 1988; Stringer et al., 1992; Kwon-Chung,
1994; Calliez et al., 1996; Stringer, 1996). Ultrastructural studies of organism morphology
indicate that different Pneumocystis species or subspecies may exist between rabbits,
rats, and mice (Nielsen et al., 1998). It is generally a harmless microorganism in
immunocompetent individuals and has been identified in clinically normal rabbits (Mata,
1959; Sheldon, 1959a; Soulez et al., 1989; Cundiff et al., 1994). Animals with a less
than fully functional immune system are susceptible to more severe infections. In
rabbits, respiratory disease accompanied by pulmonary lesions has been reported in
young or debilitated animals (Blazek, 1960; Blazek and Pokorny, 1963; Poelma and Broeckhuizen,
1972; Soulez et al., 1989). One report involving weanlings describes recovery of most
clinically affected rabbits within 2 to 3 weeks (Sheldon, 1959b). Severely affected
animals have histologic lesions characterized by extensive interstitial pneumonia
with infiltration of mononuclear cells.
F.
Management-Related Diseases
1.
Gastric Trichobezoar (Hair Ball)
The discovery of a hair ball in a rabbit is often an incidental finding during necropsy
(Fig. 11
). Indeed, up to 21% of rabbits have been found to have gastric trichobezoars during
routine necropsy (Leary et al., 1984). If the trichobezoar causes partial or complete
blockage, clinical signs of intestinal obstruction will result. Death can occur due
to prolonged anorexia and metabolic imbalances (Gillett et al., 1983). It appears
that obstruction of the pylorus, and not the volume of the gastric mass, is the critical
factor in determining the clinical progress of the animal (Leary et al., 1984). Gastric
rupture can also result from an obstructive trichobezoar (Gillett et al., 1983).
Fig. 11
Gastric trichobezoar from a rabbit. Note the large mass of hair entwined with ingesta
occupying most of the lumenal space of the stomach.
Diagnosis is often difficult because the clinical signs are nonspecific and the disease
often progresses gradually. Some cases involving acute pyloric obstruction result
in sudden clinical disease and rapid clinical decline of the animal. Manual palpation
may indicate presence of a firm mass in the cranial abdomen. Gastric radiographs using
contrast media may aid in the diagnosis, but definitive diagnosis is often made during
exploratory surgery (Gillett et al., 1983).
Treatment of trichobezoar is often unsuccessful. Oral administration of mineral oil
at 10 ml per day has been reported (Suckow and Douglas, 1997). Alternatively, oral
administration of 5–10 ml of fresh pineapple juice daily has been reported as a possible
treatment modality (Harkness and Wagner, 1995). If medical treatment does not resolve
the condition, a gastrotomy should be performed. Early surgical intervention is important
in such cases, as other, subsequent metabolic abnormalities may quickly increase the
surgical risk to the rabbit (Bergdall and Dysko, 1994).
2.
Traumatic Vertebral Fracture (Broken Back)
Subluxation or compression fractures of lumbar vertebrae are often secondary to struggling
during restraint, particularly when the hindquarters of the rabbit are not supported
(Bergdall and Dysko, 1994). The seventh lumbar vertebra (L7) or its caudal articular
processes are considered the most frequent sites of fractures, with fracture occurring
more commonly than dislocation (Flatt et al., 1974). Clinical signs include posterior
paresis or paralysis, loss of sensation in the hindlimbs, urinary and/or fecal incontinence,
and perineal staining. Diagnosis is made based on clinical signs, history of recent
restraint, struggling or other trauma, and palpation or radiographic analysis of the
vertebral column. Euthanasia of affected animals is usually warranted. Moderate cases
(subluxation with spinal edema) may resolve over time. The decision to euthanatize
should be based on severity of clinical signs. Supportive care includes regular expression
of the urinary bladder and prevention and treatment of decubital ulcers. Corticosteroid
and diuretic therapy may be effective for cases of subluxation with spinal edema (Bergdall
and Dysko, 1994).
3.
Ulcerative Pododermatitis
Although the condition is often referred to as “sore hocks,” the correct name is ulcerative
pododermatitis. Despite the name, the condition rarely affects the hocks, but rather
occurs most frequently on the plantar surface of the metatarsal and, to a lesser extent,
the metacarpal regions. The condition is believed to be initiated by wire-floor housing,
foot stomping, or having thin plantar fur pads. Poor sanitation may worsen the condition.
Larger rabbits are more commonly affected.
G.
Heritable Diseases
A number of heritable and congenital anomalies occur in rabbits. About one-third of
them are related to pelage types and colors, one-third to blood groups and tissue
antigen types, and the remainder to anatomic variants and heritable diseases. Some
of these, such as the Watanabe heritable hyperlipidemic (WHHL) rabbit, have been used
as disease research models.
Spontaneous heritable conditions in the rabbit either can involve a single gene or
can be polygenic. In addition, artificially created transgenic rabbits with disease
conditions have been developed; however, that process is beyond the scope of the current
discussion. Several of the more common heritable diseases are discussed in greater
detail below.
1.
Hydrocephalus
Hydrocephalus refers to dilatation of the cerebral ventricles and is usually accompanied
by an accumulation of cerebrospinal fluid within the dilated spaces (Fig. 12
). Some cases of hydrocephalus in rabbits have been presumed to be related to a single
autosomal recessive gene (hy/hy); however, occurrence with other abnormalities suggests
that inheritance may be more complicated (Lindsey and Fox, 1994). In some cases, the
condition appears to be inherited along with various ocular anomalies as an autosomal
gene with incomplete dominance. In addition, hydrocephalus can occur in rabbits as
a congenital condition related to hypovitaminosis A in pregnant does (Lindsey and
Fox, 1994). In contrast, the condition may also be the result of an inherited underlying
defect in vitamin A metabolism.
Fig. 12
Dorsal view of a rabbit with hydrocephalus, with top of the calvarium removed. The
ventricles are enlarged secondary to abnormal accumulation of cerebrospinal fluid.
2.
Buphthalmia (Hydrophthalmia, Congenital or Infantile Glaucoma)
Buphthalmia is inherited as an autosomal recessive trait, although penetrance is presumably
incomplete since severity and the age of onset vary greatly and some bu/bu individuals
do not develop buphthalmia (Hanna et al., 1962). The condition is common in New Zealand
White rabbits bred for research and other purposes.
Clinical signs are usually seen in individuals older than 3–4 months of age, but rabbits
that become buphthalmic demonstrate increased intraocular pressures as early as 3
months of age (Burrows et al., 1995). Animals may be affected either uni- or bilaterally.
Typical changes include increased corneal diameter (Fig. 13
), often with a cloudy or bluish tint, corneal edema, increased corneal vascularity,
and flattening of the cornea. In some cases, the cornea ulcerates and ruptures. There
is also a marked reduction in semen concentration in buphthalmics, with a decrease
in libido and decreased spermatogenesis in affected males (Fox et al., 1969).
Fig. 13
A New Zealand White rabbit with buphthalmos. Note the enlarged globe. Buphthalmos
often occurs secondary to glaucoma in the rabbit, in which it is an autosomal recessive
trait.
The condition is associated with abnormal production and removal of aqueous humor
from the anterior chamber. Impaired aqueous outflow may be due to incomplete cleavage
of the drainage angle with abnormal insertion of uveal tissue into the cornea (Tesluk
et al., 1982). Alternatively, it may be related to deposition of fibrin in the trabecular
tissue, leading to obstruction of drainage. In affected individuals, there is an absence
or underdevelopment of outflow channels of the ciliary body and sclera. By 3 months
of age, decreased aqueous outflow and increased intraocular pressure can be detected.
As the intraocular pressure increases, the globe enlarges since the scerla is still
immature. Structural changes may include widening of the angle, thickening of Descmet's
membrane, atrophy of the ciliary process, and excavation of the optic disk.
Specific treatment of buphthalmia has not been described for rabbits; however, affected
individuals should not be used for breeding purposes.
3.
Mandibular Prognathism (Malocclusion, Walrus Teeth, Buckteeth)
Mandibular prognathism is the most common inherited disease of domestic rabbits. The
condition is inherited as an autosomal recessive trait (mp/mp) with incomplete penetrance
(Fox and Crary, 1971; Huang et al., 1981; Lindsey and Fox, 1994).
Normally, the lower incisors occlude with the large upper incisors, as well as with
a pair of small secondary incisors that are immediately caudal to the primary maxillary
incisors. The lower set of incisors typically wear against the upper set during normal
biting activity, along an arc formed by biting movements of the lower incisors, while
the maxillary secondary incisors wear at right angles to the mandibular incisors.
The incisors wear more quickly at the posterior aspect in rabbits, partly because
the enamel layer is thinner on that side. Affected rabbits have a normal dental formula.
The specific abnormality associated with mandibular prognathism is that the maxilla
is short relative to a mandible of normal length. Thus, although the mandible appears
abnormally long, the primary defect involves the maxilla. In rabbits, the teeth (including
the molars and premolars) grow continuously throughout life. The incisors, for example,
grow at the rate of 2.0–2.4 mm/week. When occlusion is normal, the teeth wear against
one another and in this way remain a normal length. However, when occlusion is abnormal
because of conditions that include mandibular prognathia, the teeth may become greatly
elongated because typical attrition of the incisors does not occur. In affected animals
the lower incisors often extend anterior to the upper incisors and protrude from the
mouth, while the upper primary incisors grow past the lower incisors and curl within
the mouth (Fig. 14
). In some instances, the upper incisors curl around dorsally and lacerate the mucosa
of the hard palate. Secondary infection and abscessation may occur in such cases.
Fig. 14
Dental malocclusion involving the incisors in a rabbit. The upper incisors often curl
back into the mouth and may lacerate the hard palate. The lower incisors can curl
outward and be fractured off, leaving no evidence of malocclusion other than staining
of the chin with saliva.
Malocclusion related to mandibular prognathia may be clinically apparent as early
as 2–3 weeks of age, but is more typically seen in older rabbits. Clinical signs may
include anorexia and weight loss. If severe enough and left untreated, affected animals
will starve since they cannot properly prehend and masticate food. Overgrown teeth
should be trimmed every 2–3 weeks or more frequently if needed. Trimming is preferably
performed with a dental bur to avoid cracking the tooth, which may happen more frequently
if a bone or wire cutter is used. Care should be taken to avoid exposing the pulp
cavity as the result of excessive trimming. Because the condition is hereditary, use
of affected animals as breeding stock should be avoided.
4.
Splay Leg
A number of disorders characterized clinically by complete abduction of one or more
legs and the inability to assume a normal standing position are described by the term
“splay leg” (Fig. 15
). Young kits 3 to 4 weeks of age are most commonly affected. Affected rabbits cannot
adduct limbs and have difficulty in making normal locomotory movements. Most commonly,
animals are affected in the right rear limb, although the condition may be uni- or
bilateral and may affect the anterior, posterior, or all four limbs. Rabbits with
splay leg may have difficulty in accessing food and water; thus, attention to adequate
nutrition is required as part of a proper clinical response.
Fig. 15
Splay leg in two New Zealand White rabbits. The condition is characterized by inability
to adduct the limbs. The rabbits shown are affected in the hindlimbs only (left) and
all limbs (right).
The clinical signs of splay leg may be due to an overall imbalance of development
of the neural, muscular, and skeletal systems. Possibly, some animals compensate with
torsion and exorotation of the limb at the hip, while rabbits that are unable to compensate
are clinically affected.
Although the precise pathogenesis of splay leg is not entirely understood, at least
some cases are ascribed to inherited disorders. Typical clinical signs are secondary
to femoral endotorsion, with a shallow acetabulum but without luxation of the femur
at the hip. The semitendinosus muscle of affected animals is abnormal, with smaller
fibers and abnormal mitochondria. Some reports suggest that the condition is associated
with inherited achondroplasia of the hip and shoulder, while others indicate that
a recessively inherited anteversion of the femoral head can be involved (Lindsey and
Fox, 1974, 1994). In the Dutch breed, splay leg has been associated with either a
single recessive gene with incomplete penetrance or as a polygenic condition with
environmental modulation (Joosten et al., 1981). It has been further speculated that
some cases of splay leg are the result of teratologic malformations (Flatt et al.,
1974).
5.
Inherited Self-Mutilating Behavior
Self-mutilating behavior in a Checkered cross (cross between English Spot, German
Checkered Giant, and Checkered of Rhineland rabbits) is reported to occur as an inherited
trait (Iglauer et al., 1995). Autotraumatization of the feet and pads was observed.
The abnormal behavior could be interrupted by administration of haloperidol.
6.
Atropine Esterase Activity
Although not manifested as a disease, the presence of serum atropine esterase allows
rabbits to inactivate atropine when administered for therapeutic purposes (Stormont
and Suzuki, 1970; Liebenberg and Linn, 1979). The enzyme also permits rabbits to consume
diets containing belladonna compounds.
The enzyme is controlled by the semidominant gene Est-2¥.
Three phenotypes are recognized depending on the number of genes expressed. The enzyme
first appears in the serum at 1 month of age, and enzyme levels are greater in females
than in males (Lindsey and Fox, 1974, 1994). The Est-2F
gene is linked to genes controlling the black pigments in the coat (Sawin and Crary,
1943; Fox and van Zutphen, 1977; Forster and Hannafin, 1979).
H.
Neoplasia
Historically, spontaneous neoplasia in the laboratory rabbit has not been widely reported.
This is probably because neoplasia in the rabbit is very uncommon before 2 years of
age, and many laboratory rabbits are not maintained beyond this age. Instead, neoplasia
is more common with increasing age in rabbits, as it is in most mammals (Weisbroth,
1994). However, because of increasing use of specific pathogen-free rabbits, the use
of better feeding and husbandry practices, and the increasing tendency to maintain
antibody-producing rabbits for many years, neoplasia may actually be one of the more
common spontaneous diseases of laboratory rabbits. Table VIII
shows the rabbit tumors observed at the University of Michigan Unit for Laboratory
Animal Medicine over a period of approximately 30 years. Almost all rabbits with neoplasia
were over 2 years of age. Tumors induced by viruses are discussed under viral diseases.
Table VIII
Neoplasia in Laboratory Rabbits at the University of Michigan
Tumor type
Number
Femalea
Male
Uterine adenocarcinoma
23
23
N/A
Mammary adenocarcinoma
9
9
N/A
Malignant lymphoma
3
2
1
Basal cell tumor
3
3
0
Uterine leiomyosarcoma
2
2
N/A
Embryonal nephroma
1
0
1
Thyroid adenoma
1
1
0
Fibrosarcoma (foot)
1
1
0
Neurofibrosarcoma (foot)
1
1
0
Osteosarcoma
1
1
0
Retroperitoneal lipoma
1
1
0
Rhabdomyosarcoma (leg)
1
1
0
Squamous cell carcinoma (gingiva)
1
0
1
Testicular teratoma
1
0
1
Interstitial cell tumor
1
0
1
Total
50
45
5
a
The population evaluated consisted primarily of females, many of which were aged adults.
1.
Neoplasia of Genitourinary System and Mammary Gland
Uterine adenocarcinoma is by far the most common tumor in rabbits. Typically, the
disease is present as multiple tumors and is malignant, often metastasizing to the
liver, lungs, and other organs. There is evidence that inheritance plays a role in
susceptibility, but parity does not. Uterine leiomyomas and leiomyosarcomas (Table
VIII) (Weisbroth, 1994) are much less common. There are a few reports of vaginal squamous
cell carcinomas (Weisbroth, 1994) and an ovarian hemangioma has been described (Greene
and Strauss, 1949).
Mammary adenocarcinomas are fairly common in older female rabbits and may occur in
animals with uterine adenocarcinoma (Table VIII) (Weisbroth, 1994). Papillomas have
been described, but mammary adenocarcinomas are much more important. These malignant
tumors may metastasize, but the cause of death in affected rabbits is often due to
uterine adenocarcinoma. Serial biopsy studies indicate that these tumors are preceded
by cystic mastopathy and changes in the adrenal and pituitary glands (Greene, 1965).
Another paper has described the presence of small prolactin-secreting pituitary adenomas
in rabbits with mammary dysplasia (Lipman et al., 1994).
Testicular tumors in the rabbit appear to be relatively uncommon. Interstitial tumors
are the most common testicular tumor in the rabbit (Fig. 16
). Seminomas and teratomas have also been reported (Weisbroth, 1994).
Fig. 16
Interstitial cell tumor in the testis of a rabbit (top). Note the atrophy of the contralateral
testis (bottom).
Embryonal nephromas are one of the most common tumors in laboratory rabbits. These
tumors are often found incidentally, occur in younger animals, and seldom cause clinical
signs (Weisbroth, 1994). There has been only one report of a renal carcinoma in the
rabbit (Kaufman and Quist, 1970) and one report of a leiomyoma arising in the urinary
bladder (Weisbroth, 1974).
2.
Neoplasia of Hematopoietic System
Malignant lymphomas (lymphosarcomas) are relatively common in rabbits. They may occur
in rabbits that are less than 2 years of age (Weisbroth, 1994), but older rabbits
may also be affected (Table VIII). According to Weisbroth (1994), a tetrad of lesions
is often seen. These lesions include enlarged kidneys, splenomegaly, hepatomegaly,
and lymphadenopathy. Older rabbits have presented with skin nodules and eye lesions
(Table VIII); however, malignant lymphomas in the rabbit are seldom leukemic. Most
cases of malignant lymphoma appear to resemble the lymphoblastic subtype as seen in
humans and mice. Malignant lymphoma is more prevalent in some strains of rabbits than
others, and there is some evidence for a retroviral cause of lymphomas in rabbits
(Weisbroth, 1994). True thymomas (containing both lymphoid and epithelial components)
(Vernau et al., 1995) and plasma cell myelomas (Pascal, 1961) are rare in rabbits.
One case of myeloid leukemia has been reported (Meier et al., 1972).
3.
Neoplasia of Intestinal Tract
Bile duct adenomas and carcinomas are said to be rather common tumors in rabbits.
Weisbroth (1994) has speculated that they may be preceded by and associated with bile
duct hyperplasia induced by infection with Eimeria stiedae.
Other tumors of the intestinal tract appear to be very uncommon. These include a squamous
cell carcinoma of the gingiva (Table VIII), mucoepidermoid carcinoma (thought to be
derived from salivary gland tissue) (Gillett and Gunther, 1990), gastric adenocarcinomas
(Weisbroth, 1994), and a pancreatic adenocarcinoma (Roudebush, 1977), as well as gastric
and intestinal leiomyosarcomas (Weisbroth, 1994).
4.
Neoplasia of Skin and Subcutaneous Tissue
Basal cell tumors are reported to be rare (Weisbroth, 1994), but they may be underreported
(Table VIII) (Li and Schlafer, 1992). Squamous cell carcinomas are also uncommon,
and there is no apparent predilection for any particular area of the body (Weisbroth,
1994). Other cited skin-associated tumors include a trichoepithelioma (Altman et al.,
1978), a sebaceous gland carcinoma (Port and Sidor, 1978), and two malignant melanomas
(Hotchkiss et al., 1994).
5.
Neoplasia of Bone, Muscle, and Connective Tissue
Osteosarcomas are extremely rare in rabbits, and most have arisen in the mandible
or maxilla, with only one found in a long bone (Weisbroth, 1994) (Table VIII). No
primary tumors arising in cartilage have been described, although some of the reported
osteosarcomas have had cartilaginous elements. One tumor of skeletal muscle, a rhabdomyosarcoma,
has been seen (Table VIII). A few fibrosarcomas are cited by Weisbroth (1994) and
one fibrosarcoma involving the foot has been seen (Table VIII).
6.
Endocrine Gland Neoplasia
As with tumors of many other organs in the rabbit, reports of endocrine gland tumors
are also uncommon. Previously, only 2 pituitary gland tumors had been described (Weisbroth,
1994); however, Lipman et al. (1994), have reported a series of 9 cases in rabbits
with mammary dysplasia. Some of these tumors were microscopic. Adrenal tumors of rabbits
are rarely reported (Weisbroth, 1994), and no cases of islet cell tumors were found
in the literature. Chen (1986) has discussed the ectopic adrenal cortical nodules
found in rabbits. There has been one report of a thyroid adenocarcinoma (Dinges and
Kovac, 1972), and one thyroid adenoma has been seen (Table VIII).
7.
Miscellaneous Neoplasia
A number of other case reports of single tumors are found in the literature. These
include a peritoneal mesothelioma (Lichtensteiger and Leathers, 1987), an intracranial
teratoma (Bishop, 1978), an ependymoma (Kinkler and Jepsen, 1979), a neurofibrosarcoma
(Table VIII), two hemangiosarcomas (Pletcher and Murphy, 1984), and a malignant fibrous
histiocytoma (Yamamoto and Fujishiro, 1989). There are a few very old reports of lung
tumors dating to the first part of the twentieth century and cited by Weisbroth (1994).
8.
Neoplasia Models Derived from Rabbits
There are several tumor models in which the cells used for inoculation were originally
derived from rabbit tumors. These include the vx-2 carcinoma (Kidd and Rous, 1940),
the Brown Pearce carcinoma (Brown and Pearce, 1923), and the Greene melanoma (Greene,
1958). The vx-2 carcinoma originated in a squamous cell carcinoma in a rabbit carrying
a Shope papilloma. The most common modern use of this transplantable tumor is as a
model for the study of various cancer treatment modalities for metastatic tumors (Stetson
et al., 1991).
The Brown Pearce carcinoma arose from a tumor in a rabbit testis, but the exact tissue
of origin of the tumor was never determined. The tumor was readily transplantable
and caused stable metastases. Because some tumors regress, even after widespread metastases,
this tumor has been used as a model for the study of tumor immunology (Weisbroth,
1994). The Greene melanoma arose in the flank organ of a hamster and could be readily
transplanted to homologous hosts and some heterologous hosts, but not to the rabbit
(Greene, 1958). However, lines eventually developed that could be transplanted to
the rabbit. This transplantable tumor is commonly used as a model for the study of
the physiology of human ocular melanomas and treatment modalities for ocular melanomas
(Weisbroth, 1994).
I.
Miscellaneous Diseases
1.
Hydrometra
Hydrometra has been described as a clinical condition of rabbits. All cases were in
unmated rabbits that were used experimentally for the production of serum antibodies
(Morrell, 1989; Hobbs and Parker, 1990; Bray et al., 1991). Clinical signs include
abdominal distension and tachypnea. Cases are characterized by distension of the uterine
horns with a transudative fluid. One case was associated with uterine torsion (Hobbs
and Parker, 1990), and one case had apparently resolved with diuretic therapy, only
to return later (Bray et al., 1991).
2.
Liver Lobe Torsion
Most cases of liver lobe torsion in rabbits involve the caudate lobe (Bergdall and
Dysko, 1994), although one case report described torsion of the left hepatic lobe
(Wilson et al., 1987). Most reported cases have been incidental findings at necropsy.
In one report, a rabbit was observed to be jaundiced, anemic, and anorexic, with elevated
alanine aminotransferase. Torsion of the caudate liver lobe was seen at necropsy (Fitzgerald
and Fitzgerald, 1992).
3.
Urolithiasis
Calcium carbonate and triple phosphate crystals are present in the urine of normal
rabbits. These crystals contribute to the cloudy consistency of the urine (Williams,
1976). Uroliths may form from these crystals under certain conditions. Urolithiasis
resulting in hematuria has been described in New Zealand White rabbits (Garibaldi
et al., 1987). Predisposing factors include genetics, metabolic disturbances, nutritional
imbalance, decreased water consumption, and concurrent infections. Labranche and Renegar
(1996) reported a case of urolithiasis with hydronephrosis in a New Zealand White
rabbit. This condition must be distinguished from hematuria caused by endometrial
venous aneurysm in female rabbits (Bray et al., 1992).
4.
Lumbar Hernia
Herniation of the kidney along with perinephric fat has been reported (Suckow and
Grigdesby, 1993). The affected rabbit was clinically normal except for a subcutaneous
mass that had passed through the body wall. The precise etiology is not known, although
it was speculated that herniation might have occurred as the result of unreported
trauma.
5.
Anomalous Nasolacrimal Duct Apparatus
Occlusion of the nasolacrimal duct, presumably due to accumulation of fat droplets,
has been described as a putative cause of epiphora in some rabbits (Marini et al.,
1996). Although the obstruction occurred at the dorsal flexure, it is not clear if
this was due to congenital rather than acquired stenosis.
Related collections
Most cited references237
- Record: found
- Abstract: found
- Article: not found
Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the fungi.
Krisztián Kovács, J C Edman, Emilia M. Sogin … (1988)
- Record: found
- Abstract: found
- Article: not found
Reference range data base for serum chemistry and hematology values in laboratory animals.
- Record: found
- Abstract: found
- Article: not found
The initial impact of rabbit hemorrhagic disease on European rabbit populations in South Australia.
G Mutze, Siobhán B. Cooke, Roger Alexander (1998)