- Record: found
- Abstract: found
- Article: found
Anti-epidermal growth factor receptor treatment for patients with Neo RAS wild-type metastatic colorectal cancer: a case report of two cases
Read this article at
Abstract
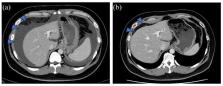
The Neo RAS phenomenon is defined as the conversion of tumor RAS status from mutant-type (MT) to wild-type (WT) after systemic chemotherapy in metastatic colorectal cancer (mCRC). Cetuximab, an anti-epidermal growth factor receptor (EGFR) antibody, is effective in patients with RAS WT mCRC but ineffective in those with RAS MT mCRC; however, its outcome in patients with Neo RAS WT mCRC is unclear. Herein, we report two cases of Neo RAS WT mCRC that responded clinically to anti-EGFR treatment. The first was a 40-year-old man with synchronous peritoneal metastatic rectosigmoid cancer. The first RAS testing on tumor tissue revealed a KRAS G12C mutation, which was converted to RAS WT after two lines of chemotherapy, as assessed by liquid biopsy. After initiating irinotecan plus cetuximab treatment, a computed tomography (CT) scan revealed that malignant ascites had resolved. The treatment was discontinued after 4 months because of disease progression. The second was a 68-year-old male patient with synchronous liver metastasis from sigmoid colon cancer. The KRAS G12D mutation, initially detected in tumor tissue, was not detected by liquid biopsy after six lines of chemotherapy. Cetuximab monotherapy was initiated, and the liver metastases shrank significantly. The patient continued cetuximab monotherapy for 8 months without disease progression. Our cases demonstrate the efficacy of anti-EGFR therapy for Neo RAS WT mCRC and highlight the importance of capturing the gene mutation profile throughout the clinical course for optimal treatment selection.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries

- Record: found
- Abstract: found
- Article: found
Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer
- Record: found
- Abstract: found
- Article: not found