- Record: found
- Abstract: found
- Article: found
Freely Behaving Mice Can Brake and Turn During Optogenetic Stimulation of the Mesencephalic Locomotor Region
Read this article at
Abstract
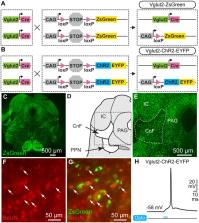
A key function of the mesencephalic locomotor region (MLR) is to control the speed of forward symmetrical locomotor movements. However, the ability of freely moving mammals to integrate environmental cues to brake and turn during MLR stimulation is poorly documented. Here, we investigated whether freely behaving mice could brake or turn, based on environmental cues during MLR stimulation. We photostimulated the cuneiform nucleus (part of the MLR) in mice expressing channelrhodopsin in Vglut2-positive neurons in a Cre-dependent manner (Vglut2-ChR2-EYFP) using optogenetics. We detected locomotor movements using deep learning. We used patch-clamp recordings to validate the functional expression of channelrhodopsin and neuroanatomy to visualize the stimulation sites. In the linear corridor, gait diagram and limb kinematics were similar during spontaneous and optogenetic-evoked locomotion. In the open-field arena, optogenetic stimulation of the MLR evoked locomotion, and increasing laser power increased locomotor speed. Mice could brake and make sharp turns (~90°) when approaching a corner during MLR stimulation in the open-field arena. The speed during the turn was scaled with the speed before the turn, and with the turn angle. Patch-clamp recordings in Vglut2-ChR2-EYFP mice show that blue light evoked short-latency spiking in MLR neurons. Our results strengthen the idea that different brainstem neurons convey braking/turning and MLR speed commands in mammals. Our study also shows that Vglut2-positive neurons of the cuneiform nucleus are a relevant target to increase locomotor activity without impeding the ability to brake and turn when approaching obstacles, thus ensuring smooth and adaptable navigation. Our observations may have clinical relevance since cuneiform nucleus stimulation is increasingly considered to improve locomotion function in pathological states such as Parkinson’s disease, spinal cord injury, or stroke.
Related collections
Most cited references93
- Record: found
- Abstract: not found
- Conference Proceedings: not found
Deep Residual Learning for Image Recognition
- Record: found
- Abstract: found
- Article: not found
DeepLabCut: markerless pose estimation of user-defined body parts with deep learning
- Record: found
- Abstract: found
- Article: not found
