- Record: found
- Abstract: found
- Article: found
Glial Tissue Mechanics and Mechanosensing by Glial Cells
Read this article at
Abstract
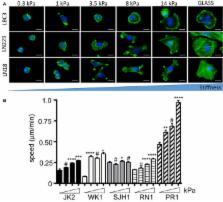
Understanding the mechanical behavior of human brain is critical to interpret the role of physical stimuli in both normal and pathological processes that occur in CNS tissue, such as development, inflammation, neurodegeneration, aging, and most common brain tumors. Despite clear evidence that mechanical cues influence both normal and transformed brain tissue activity as well as normal and transformed brain cell behavior, little is known about the links between mechanical signals and their biochemical and medical consequences. A multi-level approach from whole organ rheology to single cell mechanics is needed to understand the physical aspects of human brain function and its pathologies. This review summarizes the latest achievements in the field.
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: not found
The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells.
- Record: found
- Abstract: found
- Article: not found
Mechanical properties of gray and white matter brain tissue by indentation.
- Record: found
- Abstract: found
- Article: not found