- Record: found
- Abstract: found
- Article: found
Multidrug- and Extensively Drug-Resistant Uropathogenic Escherichia coli Clinical Strains: Phylogenetic Groups Widely Associated with Integrons Maintain High Genetic Diversity
Read this article at
Abstract
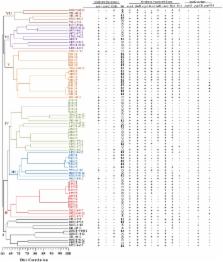
In recent years, an increase of uropathogenic Escherichia coli (UPEC) strains with Multidrug-resistant (MDR) and Extensively Drug-resistant (XDR) profiles that complicate therapy for urinary tract infections (UTIs) has been observed and has directly impacted costs and extended hospital stays. The aim of this study was to determine MDR- and XDR-UPEC clinical strains, their virulence genes, their phylogenetic groups and to ascertain their relationship with integrons and genetic diversity. From a collection of 500 UPEC strains, 103 were selected with MDR and XDR characteristics. MDR-UPEC strains were mainly associated with phylogenetic groups D (54.87%) and B2 (39.02%) with a high percentage (≥70%) of several fimbrial genes ( ecpA, fimH, csgA, and papGII), an iron uptake gene ( chuA), and a toxin gene ( hlyA). In addition, a moderate frequency (40–70%) of other genes ( iutD, tosA, and bcsA) was observed. XDR-UPEC strains were predominantly associated with phylogenetic groups B2 (47.61%) and D (42.85%), which grouped with ≥80 virulence genes, including ecpA, fimH, csgA, papGII, iutD, and chuA. A moderate frequency (40–70%) of the tosA and hlyA genes was observed. The class 1 and 2 integrons that were identified in the MDR- and XDR-UPEC strains were associated with phylogenetic groups D, B2, and A, while the XDR-UPEC strains that were associated with phylogenetic groups B2, D, and A showed an extended-spectrum beta-lactamase (ESBL) phenotype. The modifying enzymes ( aadA1, aadB, aacC, ant1, dfrA1, dfrA17, and aadA4) that were identified in the variable region of class 1 and 2 integrons from the MDR strains showed resistance to gentamycin (56.25 and 66.66%, respectively) and trimethoprim-sulfamethoxazole (84.61 and 66.66%, respectively). The MDR- and XDR-UPEC strains were distributed into seven clusters and were closely related to phylogenic groups B2 and D. The diversity analysis by PFGE showed 42.68% of clones of MDR-UPEC and no clonal association in the XDR-UPEC strains. In conclusion, phylogenetic groups including virulence genes are widely associated with two integron classes (1 and 2) in MDR- and XDR-UPEC strains.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation.

- Record: found
- Abstract: found
- Article: found
Integron Involvement in Environmental Spread of Antibiotic Resistance
- Record: found
- Abstract: found
- Article: not found