- Record: found
- Abstract: found
- Article: found
To reveal pharmacological targets and molecular mechanisms of curcumol against interstitial cystitis

Read this article at
Graphical abstract
Highlights
-
•
A PPI network showing protein interaction was produced.
-
•
3 top biotargets of curcumol against IC were identified.
-
•
Human IC sections showed increased PTK2, p-PTK2 Tyr397 expressions.
-
•
Curcumol-treated IC mice benefited reduced PTK2, p-PTK2 Tyr397 expressions.
-
•
PTK2 may be a potential biomarker for screening and treating IC.
Abstract
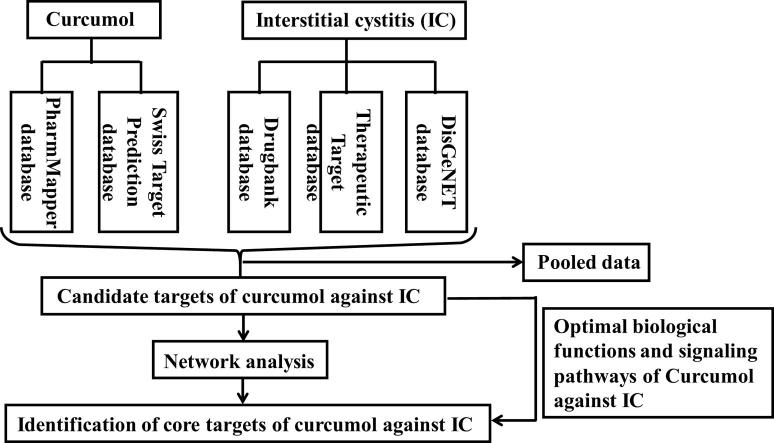
This study was designed to reveal the predictive targets and biological mechanisms of curcumol against interstitial cystitis (IC). By use of available databases and bioinformatic assays, pathogenetic targets of IC and functional targets of curcumol were identified respectively. A network of functional protein-protein interaction (PPI) was produced before screening the main predictive targets, biological processes and signaling pathways of curcumol against IC. In bioinformatic findings, the data of ingenuity pathway analysis (IPA) delineated that curcumol exerted anti-IC benefits through regulating multipronged signaling pathways, including tyrosine protein kinase-2 (PTK2) pathway. Further, optimal 18 biotargets of curcumol against IC were harvested through differential expression analysis. And the predictive targets of receptor tyrosine-protein kinase erbB-2 (ERBB2), epidermal growth factor receptor (EGFR) and PTK2 were the most important molecules. In further validated experiments, PTK2 and phosphorylation PTK2 (p-PTK2) were representatively selected for testing by human and animal IC samples. As results, increased immunoreactive proteins of tumor necrosis factor alpha (TNF-α), PTK2 and p-PTK2 Tyr397 in human IC sections were observed, accompanied with altered urinary parameters. Interestingly, curcumol-treated IC mice showed that intracellular expressions of PTK2, p-PTK2 Tyr397 in bladder samples were reduced, accompanied with lowered blood inflammatory cytokines of interleukin 6 (IL-6), TNF-α. In conclusion, the current bioinformatic data and preliminary findings unravel that the predominant targets of curcumol against IC may be the potential biological markers for screening and treating IC, such as PTK2 molecule.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
Integrin-regulated FAK-Src signaling in normal and cancer cells.
- Record: found
- Abstract: found
- Article: not found
Compromised MAPK signaling in human diseases: an update.

- Record: found
- Abstract: found
- Article: found