- Record: found
- Abstract: found
- Article: found
Myeloid-derived suppressor cell mitochondrial fitness governs chemotherapeutic efficacy in hematologic malignancies
Read this article at
Abstract
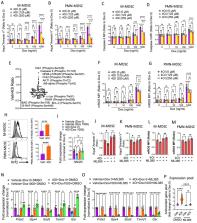
Myeloid derived suppressor cells (MDSCs) are key regulators of immune responses and correlate with poor outcomes in hematologic malignancies. Here, we identify that MDSC mitochondrial fitness controls the efficacy of doxorubicin chemotherapy in a preclinical lymphoma model. Mechanistically, we show that triggering STAT3 signaling via β2-adrenergic receptor (β2-AR) activation leads to improved MDSC function through metabolic reprograming, marked by sustained mitochondrial respiration and higher ATP generation which reduces AMPK signaling, altering energy metabolism. Furthermore, induced STAT3 signaling in MDSCs enhances glutamine consumption via the TCA cycle. Metabolized glutamine generates itaconate which downregulates mitochondrial reactive oxygen species via regulation of Nrf2 and the oxidative stress response, enhancing MDSC survival. Using β2-AR blockade, we target the STAT3 pathway and ATP and itaconate metabolism, disrupting ATP generation by the electron transport chain and decreasing itaconate generation causing diminished MDSC mitochondrial fitness. This disruption increases the response to doxorubicin and could be tested clinically.
Abstract
Myeloid derived suppressor cells (MDSC) are associated with tumourigenesis and therapy response. Here, the authors show that beta 2-adrenergic receptor activation in MDSC leads to metabolic rewiring which regulates chemotherapy response in preclinical models of blood cancer.
Related collections
Most cited references72
- Record: found
- Abstract: found
- Article: not found
AMPK: guardian of metabolism and mitochondrial homeostasis.

- Record: found
- Abstract: found
- Article: found
Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards
- Record: found
- Abstract: found
- Article: not found
