- Record: found
- Abstract: found
- Article: found
Contributions of the Hippocampal CA3 Circuitry to Acute Seizures and Hyperexcitability Responses in Mouse Models of Brain Ischemia
Read this article at
Abstract
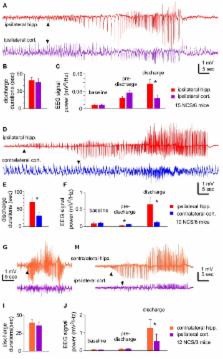
The hippocampal circuitry is widely recognized as susceptible to ischemic injury and seizure generation. However, hippocampal contribution to acute non-convulsive seizures (NCS) in models involving middle cerebral artery occlusion (MCAO) remains to be determined. To address this, we occluded the middle cerebral artery in adult C57 black mice and monitored electroencephalographic (EEG) discharges from hippocampal and neocortical areas. Electrographic discharges in the absence of convulsive motor behaviors were observed within 90 min following occlusion of the middle cerebral artery. Hippocampal discharges were more robust than corresponding cortical discharges in all seizure events examined, and hippocampal discharges alone or with minimal cortical involvement were also observed in some seizure events. Seizure development was associated with ipsilateral hippocampal injuries as determined by subsequent histological examinations. We also introduced hypoxia-hypoglycemia episodes in mouse brain slices and examined regional hyperexcitable responses ex vivo. Extracellular recordings showed that the hippocampal CA3 region had a greater propensity for exhibiting single/multiunit activities or epileptiform field potentials following hypoxic-hypoglycemic (HH) episodes compared to the CA1, dentate gyrus, entorhinal cortical (EC) or neocortical regions. Whole-cell recordings revealed that CA3 pyramidal neurons exhibited excessive excitatory postsynaptic currents, attenuated inhibitory postsynaptic currents and intermittent or repetitive spikes in response to HH challenge. Together, these observations suggest that hippocampal discharges, possibly as a result of CA3 circuitry hyperexcitability, are a major component of acute NCS in a mouse model of MCAO.
Related collections
Most cited references69
- Record: found
- Abstract: found
- Article: not found
Mechanisms of spreading depression and hypoxic spreading depression-like depolarization.

- Record: found
- Abstract: found
- Article: found
Ischemic stroke: experimental models and reality
- Record: found
- Abstract: found
- Article: not found