- Record: found
- Abstract: found
- Article: found
Microenvironmental Regulation of Tumor Progression and Therapeutic Response in Brain Metastasis
Read this article at
Abstract
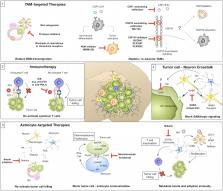
Cellular and non-cellular components of the tumor microenvironment (TME) are emerging as key regulators of primary tumor progression, organ-specific metastasis, and therapeutic response. In the era of TME-targeted- and immunotherapies, cancer-associated inflammation has gained increasing attention. In this regard, the brain represents a unique and highly specialized organ. It has long been regarded as an immunological sanctuary site where the presence of the blood brain barrier (BBB) and blood cerebrospinal fluid barrier (BCB) restricts the entry of immune cells from the periphery. Consequently, tumor cells that metastasize to the brain were thought to be shielded from systemic immune surveillance and destruction. However, the detailed characterization of the immune landscape within border-associated areas of the central nervous system (CNS), such as the meninges and the choroid plexus, as well as the discovery of lymphatics and channels that connect the CNS with the periphery, have recently challenged the dogma of the immune privileged status of the brain. Moreover, the presence of brain metastases (BrM) disrupts the integrity of the BBB and BCB. Indeed, BrM induce the recruitment of different immune cells from the myeloid and lymphoid lineage to the CNS. Blood-borne immune cells together with brain-resident cell-types, such as astrocytes, microglia, and neurons, form a highly complex and dynamic TME that affects tumor cell survival and modulates the mode of immune responses that are elicited by brain metastatic tumor cells. In this review, we will summarize recent findings on heterotypic interactions within the brain metastatic TME and highlight specific functions of brain-resident and recruited cells at different rate-limiting steps of the metastatic cascade. Based on the insight from recent studies, we will discuss new opportunities and challenges for TME-targeted and immunotherapies for BrM.
Related collections
Most cited references97
- Record: found
- Abstract: found
- Article: not found
Genes that mediate breast cancer metastasis to the brain.
- Record: found
- Abstract: found
- Article: not found
Uniquely hominid features of adult human astrocytes.
- Record: found
- Abstract: found
- Article: not found