- Record: found
- Abstract: found
- Article: found
Biology and Biomechanics of the Heart Valve Extracellular Matrix
Read this article at
Abstract
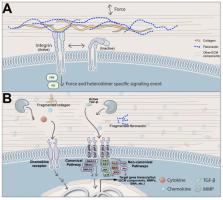
Heart valves are dynamic structures that, in the average human, open and close over 100,000 times per day, and 3 × 10 9 times per lifetime to maintain unidirectional blood flow. Efficient, coordinated movement of the valve structures during the cardiac cycle is mediated by the intricate and sophisticated network of extracellular matrix (ECM) components that provide the necessary biomechanical properties to meet these mechanical demands. Organized in layers that accommodate passive functional movements of the valve leaflets, heart valve ECM is synthesized during embryonic development, and remodeled and maintained by resident cells throughout life. The failure of ECM organization compromises biomechanical function, and may lead to obstruction or leaking, which if left untreated can lead to heart failure. At present, effective treatment for heart valve dysfunction is limited and frequently ends with surgical repair or replacement, which comes with insuperable complications for many high-risk patients including aged and pediatric populations. Therefore, there is a critical need to fully appreciate the pathobiology of biomechanical valve failure in order to develop better, alternative therapies. To date, the majority of studies have focused on delineating valve disease mechanisms at the cellular level, namely the interstitial and endothelial lineages. However, less focus has been on the ECM, shown previously in other systems, to be a promising mechanism-inspired therapeutic target. Here, we highlight and review the biology and biomechanical contributions of key components of the heart valve ECM. Furthermore, we discuss how human diseases, including connective tissue disorders lead to aberrations in the abundance, organization and quality of these matrix proteins, resulting in instability of the valve infrastructure and gross functional impairment.
Related collections
Most cited references153
- Record: found
- Abstract: found
- Article: not found
Remodelling the extracellular matrix in development and disease.
- Record: found
- Abstract: found
- Article: not found
The extracellular matrix: not just pretty fibrils.
- Record: found
- Abstract: found
- Article: not found