- Record: found
- Abstract: found
- Article: found
Bone Morphogenetic Protein (BMP) signaling in development and human diseases
Abstract
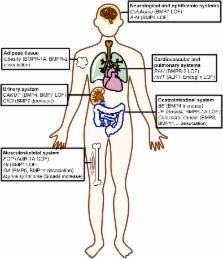
Bone Morphogenetic Proteins (BMPs) are a group of signaling molecules that belongs to the Transforming Growth Factor-β (TGF-β) superfamily of proteins. Initially discovered for their ability to induce bone formation, BMPs are now known to play crucial roles in all organ systems. BMPs are important in embryogenesis and development, and also in maintenance of adult tissue homeostasis. Mouse knockout models of various components of the BMP signaling pathway result in embryonic lethality or marked defects, highlighting the essential functions of BMPs. In this review, we first outline the basic aspects of BMP signaling and then focus on genetically manipulated mouse knockout models that have helped elucidate the role of BMPs in development. A significant portion of this review is devoted to the prominent human pathologies associated with dysregulated BMP signaling.
Related collections
Most cited references245
- Record: found
- Abstract: found
- Article: not found
New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure.
- Record: found
- Abstract: found
- Article: not found
Novel regulators of bone formation: molecular clones and activities.
- Record: found
- Abstract: found
- Article: not found