- Record: found
- Abstract: found
- Article: found
The Histone Deacetylase Inhibitor Valproic Acid Exerts a Synergistic Cytotoxicity with the DNA-Damaging Drug Ellipticine in Neuroblastoma Cells
Read this article at
Abstract
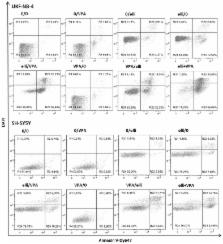
Neuroblastoma (NBL) originates from undifferentiated cells of the sympathetic nervous system. Chemotherapy is judged to be suitable for successful treatment of this disease. Here, the influence of histone deacetylase (HDAC) inhibitor valproate (VPA) combined with DNA-damaging chemotherapeutic, ellipticine, on UKF-NB-4 and SH-SY5Y neuroblastoma cells was investigated. Treatment of these cells with ellipticine in combination with VPA led to the synergism of their anticancer efficacy. The effect is more pronounced in the UKF-NB-4 cell line, the line with N-myc amplification, than in SH-SY5Y cells. This was associated with caspase-3-dependent induction of apoptosis in UKF-NB-4 cells. The increase in cytotoxicity of ellipticine in UKF-NB-4 by VPA is dictated by the sequence of drug administration; the increased cytotoxicity was seen only after either simultaneous exposure to these drugs or after pretreatment of cells with ellipticine before their treatment with VPA. The synergism of treatment of cells with VPA and ellipticine seems to be connected with increased acetylation of histones H3 and H4. Further, co-treatment of cells with ellipticine and VPA increased the formation of ellipticine-derived DNA adducts, which indicates an easier accessibility of ellipticine to DNA in cells by its co-treatment with VPA and also resulted in higher ellipticine cytotoxicity. The results are promising for in vivo studies and perhaps later for clinical studies of combined treatment of children suffering from high-risk NBL.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Extraction, purification and analysis of histones.
- Record: found
- Abstract: found
- Article: not found
The xCELLigence system for real-time and label-free monitoring of cell viability.
- Record: found
- Abstract: found
- Article: not found