- Record: found
- Abstract: found
- Article: found
Annexin A1 is elevated in patients with COPD and affects lung fibroblast function
Abstract
Purpose
Fibrosis in peripheral airways is responsible for airflow limitation in chronic obstructive pulmonary disease (COPD). Annexin A1 modulates several key biological events during inflammation. However, little is known about its role in airway fibrosis in COPD. We investigated whether levels of Annexin A1 were upregulated in patients with COPD, and whether it promoted airway fibrosis.
Methods
We quantified serum Annexin A1 levels in never-smokers (n=12), smokers without COPD (n=11), and smokers with COPD (n=22). Correlations between Annexin A1 expression and clinical indicators (eg, lung function) were assessed. In vitro, human bronchial epithelial (HBE) cells were exposed to cigarette smoke extract (CSE) and Annexin A1 expression was assessed. Primary human lung fibroblasts were isolated from patients with COPD and effects of Annexin A1 on fibrotic deposition of lung fibroblasts were evaluated.
Results
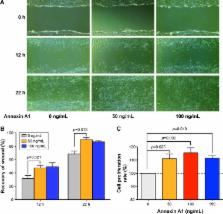
Serum Annexin A1 was significantly higher in patients with Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines stage III or IV than in those with GOLD stages I or II (12.8±0.8 ng/mL versus 9.8±0.7 ng/mL; p=0.016). Annexin A1 expression was negatively associated with airflow obstruction (forced expiratory volume in one second % predicted; r=−0.72, p<0.001). In vitro, Annexin A1 was significantly increased in CSE-exposed HBE cells in a time- and concentration-dependent manner. Annexin A1 promoted lung fibroblasts proliferation, migration, differentiation, and collagen deposition via the ERK1/2 and p38 mitogen-activated protein kinase pathways.
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair.
- Record: found
- Abstract: found
- Article: not found
