- Record: found
- Abstract: found
- Article: not found
Associations of Peripubertal Serum Dioxin and Polychlorinated Biphenyl Concentrations with Pubertal Timing among Russian Boys
Read this article at
Abstract
Background:
Dioxins, furans, and polychlorinated biphenyls (PCBs), dioxin-like and non-dioxin-like, have been linked to alterations in puberty.
Objectives:
We examined the association of peripubertal serum levels of these compounds [and their toxic equivalents (TEQs)] with pubertal onset and maturity among Russian boys enrolled at ages 8–9 years and followed prospectively through ages 17–18 years.
Methods:
At enrollment, 473 boys had serum dioxin-like compounds and PCBs measured. At the baseline visit and annually until age 17–18 years, a physician performed pubertal staging [genitalia (G), pubarche (P), and testicular volume (TV)]. Three hundred fifteen subjects completed the follow-up visit at 17–18 years of age. Pubertal onset was defined as TV > 3 mL, G2, or P2. Sexual maturity was defined as TV ≥ 20 mL, G5, or P5. Multivariable interval-censored models were used to evaluate associations of lipid-standardized concentrations with pubertal timing.
Results:
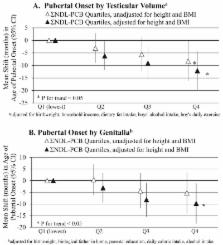
Medians (interquartile ranges) of the sum of dioxin-like compounds, TEQs, and non-dioxin-like PCBs were 362 pg/g lipid (279–495), 21.1 pg TEQ/g lipid (14.4–33.2), and 250 ng/g lipid (164–395), respectively. In adjusted models, the highest compared to lowest TEQ quartile was associated with later pubertal onset [TV = 11.6 months (95% CI: 3.8, 19.4); G2 = 10.1 months (95% CI: 1.4, 18.8)] and sexual maturity [TV = 11.6 months (95% CI: 5.7, 17.6); G5 = 9.7 months (95% CI: 3.1, 16.2)]. However, the highest compared to the lowest quartile of non-dioxin-like PCBs, when co-adjusted by TEQs, was associated with earlier pubertal onset [TV = –8.3 months (95% CI:–16.2, –0.3)] and sexual maturity [TV = –6.3 months (95% CI:–12.2, –0.3); G5 = –7.2 months (95% CI:–13.8, –0.6)]; the non-dioxin-like PCB associations were only significant when adjusted for TEQs. TEQs and PCBs were not significantly associated with pubic hair development.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds.
- Record: found
- Abstract: found
- Article: not found
Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings.
- Record: found
- Abstract: found
- Article: not found
