- Record: found
- Abstract: found
- Article: found
Role of animal models in glaucoma research
other
09 January 2020
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Glaucoma is a neurodegenerative disease of the eye, and it presents with visual field
defects accompanied by progressive degeneration of the optic nerve and retinal ganglion
cells (RGCs). It is one of the major causes of blindness worldwide and affects 1 in
20 people over the age of 40. Glaucoma is caused by multiple factors, but it is usually
associated with elevated intraocular pressure (IOP). Extensive studies have been carried
out to discover therapeutic targets and to develop new drugs to treat ocular hypertension
using experimental models of rodents (rats and mice) and non-human primates (e.g.,
cynomologus, rhesus, or marmoset monkeys), in which non-human primates provide unique
insights into disease pathology that cannot be studied in rodents. Currently, almost
all effective therapies in clinics aim to reduce IOP. However, not all glaucoma patients
respond to this type of treatment and there is a subtype of glaucoma termed normal
tension glaucoma (NTG), which is not accompanied by high IOP. Therefore, therapies
targeting factors other than IOP are unmet medical need that could benefit cases when
IOP reduction is not effective.
We have recently reported that different aspects of pathogenesis independent of IOP
are demonstrated in two types of mouse glaucoma models (Sano et al., 2019) and that
naturally occurring NTG-like neurodegeneration is observed in aged marmosets (Noro
et al., 2019). In this perspective, we discuss various mouse models of glaucoma and
the potential role of marmosets in glaucoma research.
Mouse models of glaucoma: There are a number of mouse models of glaucoma including
high IOP models, NTG models, experimentally induced models and spontaneous models
(Harada et al., 2019). The advantages of using experimentally-inducible models are
that wild-type mice can be used and experimental conditions can be carefully controlled;
for example the onset of disease. Experimentally-inducible models for high IOP glaucoma
include laser treatment to damage the trabecular meshwork, microbead injection into
the anterior chamber, cauterization of episcleral veins, and hypertonic saline injection
into the episcleral veins; these methods aim to block the aqueous outflow leading
to increased IOP. For NTG, examples include optic nerve injury, in which the optic
nerve is crushed or transected, and intravitreal injection of N-methyl-D-aspartate;
these methods cause acute RGC death independently of IOP. Although these experimental
NTG models show RGC death, one may question if they are merely RGC death models because
optic nerve injury does not necessarily cause glaucoma and there is no evidence to
show increased N-methyl-D-aspartate receptor activation in RGCs of NTG patients.
The most widely characterized spontaneous model is DBA/2J mice, which present with
a pigmentary form of glaucoma demonstrated with elevated IOP and optic nerve degeneration
(Chang et al., 1999). Other spontaneous models with increased IOP include the pyrimidinergic
receptor P2Y6 knockout (KO) mice that present with age-dependent optic nerve and RGC
degeneration accompanied by impaired visual function, due to excess production of
aqueous humor from the ciliary body (Shinozaki et al., 2017); and Vav2/Vav3 KO mice
demonstrating RGC loss and optic nerve head cupping with age, due to progressive iridocorneal
angle closure (Fujikawa et al., 2010).
Spontaneous models for NTG include overexpression of the mutated genes associated
with human NTG: optineurin E50K (Chi et al., 2010) and tank-binding protein 1 (Fingert
et al., 2017). These models recapitulate a population of human glaucoma both genetically
and phenotypically, but the late onset of disease (over 6–18 months of age) may not
be very practical from an experimental point of view. In this respect, we have reported
that mice deficient in glutamate transporters (GLAST or EAAC1) show NTG-like retinal
degeneration from 3 or 5 weeks of age, respectively (Harada et al., 2007). Although
the genetic association of GLAST or EAAC1 mutations with human glaucoma is yet to
be established, the early onset of disease in these models is helpful for experiments
on identifying therapeutic targets and interventions.
Multiple factors are involved in the pathogenesis of glaucoma, and although there
are a number of mouse glaucoma models, each represents different pathological aspects.
In humans, the lamina cribrosa (LC) is considered to be a putative site of optic nerve
damage that causes characteristic pathology of glaucoma. In mice, this tissue is absent.
Naturally, the features that cannot be reproduced in mice should be examined using
other animal models. In the case of the LC, non-human primates may be ideal. Therefore,
while taking advantage of the fast life cycle of mice to advance understanding in
medicine, use of other animal models should be considered to unravel disease pathogenesis
from a different perspective.
N-acetylcysteine (NAC) prevents retinal degeneration in EAAC1 KO mice, but not in
GLAST KO mice: Drug repositioning is an application of an existing drug to treat a
different disease. One of the main advantages of drug repositioning is that it can
save time and cost that is required to establish the safety of the drug. NAC is a
N-acetyl derivative of cysteine that has historically been used as an antidote against
paracetamol overdose, and more recently for various medical conditions including bronchopulmonary
disorders, renal disorders and neurological and psychiatric disorders. It is liposoluble,
so it can permeate across the cell membranes, and after entering the cells, it can
be rapidly hydrolyzed and converted to cysteine. In neurons, the availability of cysteine
is the rate-limiting substrate for the synthesis of glutathione (GSH), a powerful
antioxidant, so supply of cysteine can increase GSH levels that may lead to neuroprotection.
We have recently reported that daily NAC administration in EAAC1 and GLAST KO mice
have differential effects on NTG-like retinal degeneration (Sano et al., 2019). We
found that NAC administration protected RGCs in EAAC1 KO mice by increasing retinal
GSH levels and reducing 4-HNE, an oxidative stress marker, but it failed to protect
RGCs in GLAST KO mice. It was surprising to find such distinctive differences in the
two models, because they both lack glutamate transporters, though different subtypes.
EAAC1 is expressed in neurons and is involved in neuronal uptake of cysteine and glutamate.
GLAST is mainly expressed in Müller glia in the retina and it plays a major role in
removing excess glutamate, thereby protecting RGCs from glutamate neurotoxicity. Therefore,
we speculated that in EAAC1 KO mice, RGCs die mainly because of the increased oxidative
stress levels caused by the inability of RGCs to take up cysteine that is required
for GSH synthesis. Supplementation of cysteine in neurons via NAC in EAAC1 KO mice
restores the retinal GSH levels (Sano et al., 2019), and thus NAC exerts neuroprotective
effects in this mouse model. On the other hand, we speculated that in GLAST KO mice,
RGCs die mainly because of the increased glutamate neurotoxicity caused by the lack
of glutamate removal from the extracellular space. Therefore, supplementation of cysteine
via NAC could not prevent RGC death. Oxidative stress and glutamate neurotoxicity
are both potentially involved in the pathogenesis of glaucoma. These findings demonstrated
that EAAC1 and GLAST KO mice may represent different aspects of glaucoma pathogenesis
and proved that they are both independently very useful models for glaucoma.
Aged marmosets present with naturally occurring NTG: The common marmoset (Callithrix
jacchus), a small new world primate, is becoming increasingly attractive as an experimental
animal model, particularly in neuroscience research. Like humans, the common marmoset
is diurnal, and its brain and eyes are structurally well developed. The advantages
of using the common marmoset over other non-human primates include (i) a high reproduction
rate for a primate: their gestation period is about 5 months and multiple births are
common; (ii) rapid postnatal development: they reach sexual maturation at 12 to 18
months of age; and (iii) ease of handling and breeding in laboratories. Common occurrence
of multiple births is particularly useful for therapeutic studies as it enables direct
comparison of the effects of treatment and placebo between littermates. In addition,
their compact lifespan allows monitoring of aging or progressive disease effects in
a relatively short period of time, suggesting it is a good model for aging research.
Excitingly, generation of the transgenic marmoset was first reported in 2009 (Sasaki
et al., 2009) and this technology provides a powerful tool for advances in medical
research for various diseases.
We have recently reported that aged marmosets show glaucoma-like retinal and brain
degeneration as well as the thinning of the LC (Noro et al., 2019). These marmosets
had no genetic mutations in glaucoma-associated genes and no elevated IOP, suggesting
that they show naturally occurring NTG (
Figure 1
). We used a number of in vivo imaging techniques including spectral-domain optical
coherence tomography, multifocal electroretinogram and magnetic resonance imaging,
for assessment of glaucomatous pathology in marmosets to follow up disease progression
and to minimize animal sacrifice. We also demonstrated that increased oxidative stress
and reduced brain-derived neurotrophic factor levels are observed in marmosets with
glaucoma-like features. Brain-derived neurotrophic factor is a powerful neuroprotective
agent especially for RGCs and its expression is reduced in glaucoma patient eyes (Gupta
et al., 2014; Kimura et al., 2016). In addition, we found that the rate of incidence
was 11%, which is similar to human glaucoma. However, with many aging research using
non-human primates, if it takes decades before age-related conditions are apparent,
one study could extend beyond a typical scientific career. Moreover, such long-term
studies are extremely expensive, because maintenance for non-human primates requires
specialized facilities and staff. To this end, we are generating genetically modified
marmosets with early onset of disease as a marmoset model of glaucoma. Our target
gene is GLAST. Based on our mouse studies, we believe that the onset of retinal degeneration
in GLAST KO marmosets will be within a few months rather than years, and thus, they
will be a workable model that could contribute to advances in glaucoma therapy.
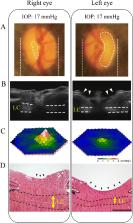
Figure 1
Eye examination of an aged marmoset with glaucoma-like degeneration (female, aged
12 years).
(A) Ocular fundus photographs. The edge of the cupping was traced from the three-dimensional
images of the optic nerve head obtained by SD-OCT and the lines were superimposed
on the fundus photograph. (B) In vivo imaging of the optic disc by vertical scan through
the centre of the optic disc by SD-OCT. Arrowheads indicate the cupping of the optic
disc and dotted lines indicate the LC. (C) Three-dimensional plots of the retinal
responses as examined by multifocal electroretinogram. A higher score (white) indicates
highly sensitive visual function. (D) Haematoxylin and eosin staining of the optic
nerve head. Enhanced optic disc cupping (arrowheads) and thinning of the LC (dotted
lines) are apparent in the left eye. Scale bar: 200 µm. Reproduced with some modification
from Noro et al. (2019). IOP: Intraocular pressure; LC: lamina cribrosa; SD-OCT: spectral-domain
optical coherence tomography.
Conclusions and future perspectives: Glaucoma is an age-related disease, and recent
drastic increase in life expectancy means that the number of glaucoma patients is
also expected to rise. Animal models of glaucoma provide useful information on the
pathogenesis and potential therapeutic targets for glaucoma, but we are still searching
for a cure and the current therapies are limited to prevent or slow down the disease
progression. Medicine is progressing and animal models are representing disease features
closer to humans than before. As a result, novel therapeutic strategies in addition
to reducing IOP are emerging. For example, delivery of a ciliary neurotrophic factor
into the eye by implanting encapsulated human cells that are genetically modified
to secrete therapeutic doses of ciliary neurotrophic factor is currently under clinical
trials for glaucoma (ClinicalTrials.gov number, NCT01408472). Many studies with animal
models pointed to the direction that neuroprotective effects of ciliary neurotrophic
factor (and other agents) may be therapeutically useful for glaucoma, and success
of this trial will prove that neuroprotection is effective in treatment of glaucoma.
In summary, it is important to understand what each animal model offers, because there
is no one model that represents the whole aspects of human glaucoma. Use of marmosets
in glaucoma research may provide further insights into the molecular mechanisms involved
in the onset and progression of glaucoma.
This work was supported by JSPS KAKENHI Grants-in-Aid for Scientific Research (JP17K07123
to AK, JP17K11499 to TN and JP18K19625 to TH), the Taiju Life Social Welfare Foundation
(to TH) and the Takeda Science Foundation (to TH).
Related collections
Most cited references10
- Record: found
- Abstract: found
- Article: not found
The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma.
Hun-Meng Quah, Takayuki Harada, Akinori Okumura … (2007)
- Record: found
- Abstract: found
- Article: found
Neuroprotection, Growth Factors and BDNF-TrkB Signalling in Retinal Degeneration
Atsuko Kimura, Kazuhiko Namekata, Xiaoli Guo … (2016)
- Record: found
- Abstract: found
- Article: not found
BDNF impairment is associated with age-related changes in the inner retina and exacerbates experimental glaucoma.
Stuart Graham, Vivek Gupta, Nai-Chieh Y You … (2014)