- Record: found
- Abstract: found
- Article: found
ER Stress and the UPR in Shaping Intestinal Tissue Homeostasis and Immunity
Read this article at
Abstract
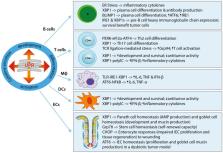
An imbalance in the correct protein folding milieu of the endoplasmic reticulum (ER) can cause ER stress, which leads to the activation of the unfolded protein response (UPR). The UPR constitutes a highly conserved and intricately regulated group of pathways that serve to restore ER homeostasis through adaptation or apoptosis. Numerous studies over the last decade have shown that the UPR plays a critical role in shaping immunity and inflammation, resulting in the recognition of the UPR as a key player in pathological processes including complex inflammatory, autoimmune and neoplastic diseases. The intestinal epithelium, with its many highly secretory cells, forms an important barrier and messenger between the luminal environment and the host immune system. It is not surprising, that numerous studies have associated ER stress and the UPR with intestinal diseases such as inflammatory bowel disease (IBD) and colorectal cancer (CRC). In this review, we discuss our current understanding of the roles of ER stress and the UPR in shaping immune responses and maintaining tissue homeostasis. Furthermore, the role played by the UPR in disease, with emphasis on IBD and CRC, is described here. As a key player in immunity and inflammation, the UPR has been increasingly recognized as an important pharmacological target in the development of therapeutic strategies for immune-mediated pathologies. We summarize available strategies targeting the UPR and their therapeutic implications. Understanding the balance between homeostasis and pathophysiology, as well as means of manipulating this balance, provides an important avenue for future research.
Related collections
Most cited references127
- Record: found
- Abstract: found
- Article: not found
Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease
- Record: found
- Abstract: found
- Article: not found
ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs.
- Record: found
- Abstract: found
- Article: not found