- Record: found
- Abstract: found
- Article: found
Pain relief in a neuropathy patient by lacosamide: Proof of principle of clinical translation from patient-specific iPS cell-derived nociceptors

Read this article at
Abstract
Background
Small fiber neuropathy (SFN) is a severe and disabling chronic pain syndrome with no causal and limited symptomatic treatment options. Mechanistically based individual treatment is not available. We report an in-vitro predicted individualized treatment success in one therapy-refractory Caucasian patient suffering from SFN for over ten years.
Methods
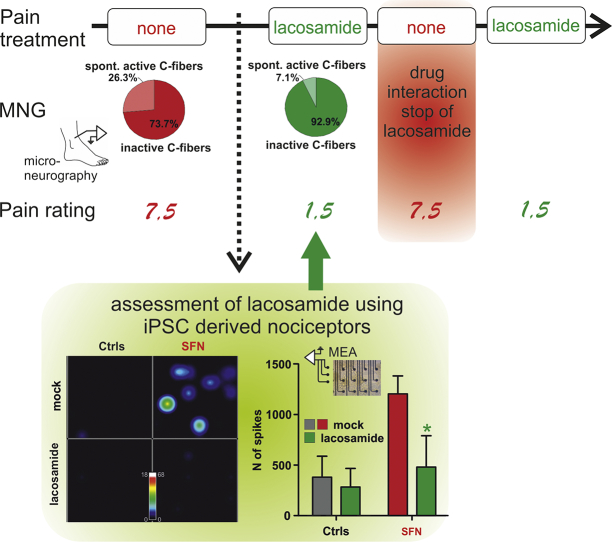
Intrinsic excitability of human induced pluripotent stem cell (iPSC) derived nociceptors from this patient and respective controls were recorded on multi-electrode (MEA) arrays, in the presence and absence of lacosamide. The patient's pain ratings were assessed by a visual analogue scale (10: worst pain, 0: no pain) and treatment effect was objectified by microneurography recordings of the patient's single nerve C-fibers.
Findings
We identified patient-specific changes in iPSC-derived nociceptor excitability in MEA recordings, which were reverted by the FDA-approved compound lacosamide in vitro . Using this drug for individualized treatment of this patient, the patient's pain ratings decreased from 7.5 to 1.5. Consistent with the pain relief reported by the patient, microneurography recordings of the patient's single nerve fibers mirrored a reduced spontaneous nociceptor (C-fiber) activity in the patient during lacosamide treatment. Microneurography recordings yielded an objective measurement of altered peripheral nociceptor activity following treatment.
Graphical abstract
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: not found
Combined small molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors
- Record: found
- Abstract: found
- Article: not found
Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures.
- Record: found
- Abstract: found
- Article: not found