- Record: found
- Abstract: found
- Article: found
Transportan-derived cell-penetrating peptide delivers siRNA to inhibit replication of influenza virus in vivo
Abstract
Introduction
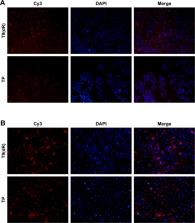
In this study, we report on the development of an effective delivery system for siRNAs; a novel cell-penetrating peptide (CPP), T9(dR), obtained from transportan (TP), was used for in vivo and in vitro testing.
Methods
In this study, toxicity of T9(dR) and TP and efficient delivery of siRNA were tested in 293T, MDCK, RAW, and A549 cells. Furthermore, T9(dR)- and TP-delivered siRNAs against nucleoprotein (NP) gene segment of influenza virus (siNP) were studied in both cell lines and mice.
Results
Gel retardation showed that T9(dR) effectively condensed siRNA into nanoparticles sized between 350 and 550 nm when the mole ratio of T9(dR) to siRNA was ≥4:1. In vitro studies demonstrated that T9(dR) successfully delivered siRNA with low cellular toxicity into several cell lines. It was also observed that T9(dR)-delivered siRNAs inhibited replication of influenza virus more efficiently as compared to that delivered by TP into the MDCK and A549 cells. It was also noticed that when given a combined tail vein injection of siNP and T9(dR) or TP, all mice in the 50 nmol siNP group infected with PR8 influenza virus survived and showed weight recovery at 2 weeks post-infection.
Most cited references44
- Record: found
- Abstract: found
- Article: not found
RNA interference of influenza virus production by directly targeting mRNA for degradation and indirectly inhibiting all viral RNA transcription.
- Record: found
- Abstract: found
- Article: not found
Progress in developing cationic vectors for non-viral systemic gene therapy against cancer.
- Record: found
- Abstract: found
- Article: not found