- Record: found
- Abstract: found
- Article: found
Stem Cell‐Derived Extracellular Vesicles as a Novel Potential Therapeutic Tool for Tissue Repair
Read this article at
Summary
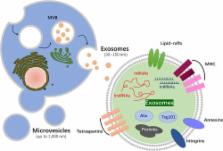
Stem cells, with their therapeutic potential in tissue repair and regeneration, have been widely used in translational medicine. Recent evidence suggests that the beneficial effects are mediated largely by their paracrine actions rather than the engraftment and differentiation at the injured sites. Extracellular vesicles (EVs), actively released from cells, play important roles in cell‐to‐cell communication and display multiple functions in tissue regeneration. In the present report, we will briefly review the current knowledge related to the therapeutic potential of EVs, particularly stem cell or progenitor cell‐derived ones for promoting tissue repair and regeneration, and focus on the restorative properties of exosomes/microvesicles in cutaneous wound healing, bone regeneration, hindlimb ischemia, and vascular injury repair. S tem C ells T ranslational M edicine 2017;6:1753–1758
Related collections
Most cited references33

- Record: found
- Abstract: found
- Article: found
Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis
- Record: found
- Abstract: found
- Article: not found
Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling.
- Record: found
- Abstract: found
- Article: not found