- Record: found
- Abstract: found
- Article: found
Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition
ABSTRACT
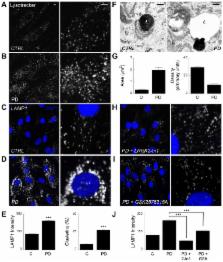
Two-pore channels (TPCs) are endolysosomal ion channels implicated in Ca 2+ signalling from acidic organelles. The relevance of these ubiquitous proteins for human disease, however, is unclear. Here, we report that lysosomes are enlarged and aggregated in fibroblasts from Parkinson disease patients with the common G2019S mutation in LRRK2. Defects were corrected by molecular silencing of TPC2, pharmacological inhibition of TPC regulators [Rab7, NAADP and PtdIns(3,5) P 2] and buffering local Ca 2+ increases. NAADP-evoked Ca 2+ signals were exaggerated in diseased cells. TPC2 is thus a potential drug target within a pathogenic LRRK2 cascade that disrupts Ca 2+-dependent trafficking in Parkinson disease.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
A gene network regulating lysosomal biogenesis and function.
- Record: found
- Abstract: found
- Article: not found
Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology.
- Record: found
- Abstract: found
- Article: not found