- Record: found
- Abstract: found
- Article: found
Signaling through the Phosphatidylinositol 3-Kinase (PI3K)/Mammalian Target of Rapamycin (mTOR) Axis Is Responsible for Aerobic Glycolysis mediated by Glucose Transporter in Epidermal Growth Factor Receptor ( EGFR)-mutated Lung Adenocarcinoma*
Read this article at
Abstract
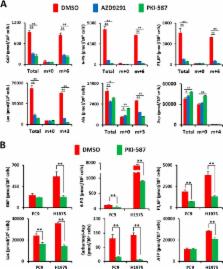
Background: EGFR signaling maintains aerobic glycolysis, but the molecular mechanism is still undefined.
Results: Drug inhibition studies reveal that downstream signaling via the PI3K pathway is critical for glucose transport and metabolism.
Conclusion: The PI3K signaling regulates key metabolic activities in EGFR-mutant lung adenocarcinoma.
Significance: These data may guide the development of chemotherapeutic options, including targeting of the PI3K pathway and glucose transporter machinery.
Abstract
Oncogenic epidermal growth factor receptor (EGFR) signaling plays an important role in regulating global metabolic pathways, including aerobic glycolysis, the pentose phosphate pathway (PPP), and pyrimidine biosynthesis. However, the molecular mechanism by which EGFR signaling regulates cancer cell metabolism is still unclear. To elucidate how EGFR signaling is linked to metabolic activity, we investigated the involvement of the RAS/MEK/ERK and PI3K/AKT/mammalian target of rapamycin (mTOR) pathways on metabolic alteration in lung adenocarcinoma (LAD) cell lines with activating EGFR mutations. Although MEK inhibition did not alter lactate production and the extracellular acidification rate, PI3K/mTOR inhibitors significantly suppressed glycolysis in EGFR-mutant LAD cells. Moreover, a comprehensive metabolomics analysis revealed that the levels of glucose 6-phosphate and 6-phosphogluconate as early metabolites in glycolysis and PPP were decreased after inhibition of the PI3K/AKT/mTOR pathway, suggesting a link between PI3K signaling and the proper function of glucose transporters or hexokinases in glycolysis. Indeed, PI3K/mTOR inhibition effectively suppressed membrane localization of facilitative glucose transporter 1 (GLUT1), which, instead, accumulated in the cytoplasm. Finally, aerobic glycolysis and cell proliferation were down-regulated when GLUT1 gene expression was suppressed by RNAi. Taken together, these results suggest that PI3K/AKT/mTOR signaling is indispensable for the regulation of aerobic glycolysis in EGFR-mutated LAD cells.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: found
The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes.
- Record: found
- Abstract: found
- Article: found
Glucose-Independent Glutamine Metabolism via TCA Cycling for Proliferation and Survival in B Cells
- Record: found
- Abstract: found
- Article: found