- Record: found
- Abstract: found
- Article: found
Representation of sex, race, and ethnicity in pivotal clinical trials for dermatological drugs
Read this article at
Abstract
Background
It is of paramount importance that clinical trials are designed with adequate health equity considerations to prevent disproportionate analyses of specific demographics.
Objective
In this study, we investigated the representation of sex, race, and ethnicity in pivotal clinical trials for drugs with dermatological disease indications approved by the U.S. Food and Drug Administration between 1995 and 2019.
Methods
Thirty-six novel drugs with indications to treat dermatological diseases, approved by the U.S. Food and Drug Administration between January 1995 and December 2019 were abstracted from Drugs@FDA. The drug approval label, statistical review, official record, and trial publication were reviewed for data on disease indication, approval year, pathway, number of participants, participant demographics (sex, race, and ethnicity), location, and sponsor type.
Results
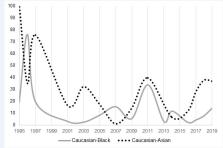
The overall female representation was 45.6% (n = 17,492 of 38,320). Adequate female representation was noted for five of six disease indications. Caucasians were predominantly overrepresented (80.4%; n = 28,065 of 34,890); Blacks (9.8%; n = 3242 of 33,240) and Asians (5.5%; n = 1535 of 27,696) were consistently underrepresented. Across sponsor types, there was a significant difference in the distribution of women (χ 2 = 6.332; p = .042), as well as Caucasians (χ 2 = 12.813; p = .002), Blacks (χ 2 = 13.002; p = .002) , and Hispanics/Latinos (χ 2 = 7.747; p = .021).
Conclusion
Persistence of disparities disproportionately affect the quality of data behind therapies for certain demographics; as such, enrollment practices must continue to address the issue of underrepresentation. Efforts to facilitate demographic equity among clinical trial participants must be supported to ensure that safety and efficacy conclusions are drawn from representative population samples.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
Increasing Diversity in Clinical Trials: Overcoming Critical Barriers
- Record: found
- Abstract: found
- Article: not found
Effective recruitment and retention of minority research participants.
- Record: found
- Abstract: found
- Article: not found