- Record: found
- Abstract: found
- Article: found
Therapeutic potential of CKD-506, a novel selective histone deacetylase 6 inhibitor, in a murine model of rheumatoid arthritis
Read this article at
Abstract
Objectives
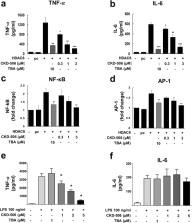
Histone deacetylase (HDAC) 6 promotes inflammation. We investigated the anti-arthritic effects of CKD-506, a novel HDAC6 inhibitor, in vitro and in a murine model of arthritis as a novel treatment option for rheumatoid arthritis (RA).
Methods
HDAC6 was overexpressed in mouse peritoneal macrophages and RAW 264.7 cells, and the effects of a HDAC6 inhibitor CKD-506 on cytokine production and activity of NF-κB and AP-1 signaling were examined. Peripheral blood mononuclear cells (PBMCs) from RA patients and fibroblast-like synoviocytes (FLS) were activated in the presence of CKD-506. Next, regulatory T cells (Tregs) were induced from RA patients and co-cultured with healthy effector T cells (Teffs) and cell proliferation was analyzed by flow cytometry. Finally, the effects of the inhibitor on the severity of arthritis were assessed in a murine model of adjuvant-induced arthritis (AIA).
Results
Overexpression of HDAC6 induced macrophages to produce TNF-α and IL-6. The inhibitory effect of CKD-506 was mediated via blockade of NF-κB and AP-1 activation. HDAC6 inhibition reduced TNF-α and IL-6 production by activated RA PBMCs. CKD-506 inhibited production of MMP-1, MMP-3, IL-6, and IL-8 by activated FLS. In addition, CKD-506 inhibited proliferation of Teffs directly and indirectly by improving iTreg function. In AIA rats, oral CKD-506 improved clinical arthritis in a dose-dependent manner. A combination of sub-therapeutic CKD-506 and methotrexate exerted a synergistic effect.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention.
- Record: found
- Abstract: found
- Article: not found