- Record: found
- Abstract: found
- Article: not found
First Cases of COVID-19 in Heart Transplantation From China
letter
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Emerging studies suggest that the novel coronavirus SARS-CoV-2 and the disease it
causes, COVID-19, selectively afflicts the elderly, particularly those with chronic
comorbidities.
1
,
2
Here, we report on two heart transplant recipients with COVID-19, one a severe presentation
and another mild.
The first was a 51-year-old man with a heart transplant on November 8th, 2003. His
immunosuppression was tacrolimus 1 mg twice daily plus mycophenolate mofetil 0.5 g
twice daily. The last known blood concentration of tacrolimus was 6.5 ng/ml, and cardiac
allograft function was normal with a history of well controlled hypertension. On Jan
23rd 2020, he complained of intermittent fever, chills, fatigue, poor appetite and
diarrhea. On examination, his temperature was 38.5°C, oxygen saturation was 99% on
room air, respiratory rate of 20 breaths per minute without distress. Laboratory tests
showed a white blood cell count of 4.87 × 109 /L (normal range, 4.0–10.0 × 109 /L),
C Reactive Protein of 18.6 mg/L (normal range, 0-10 mg/L). Chest computed tomographic
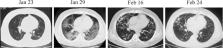
(CT) scan showed bilateral ground-glass opacities (Figure 1
). He was initially treated with intravenous levofloxacin and ribavirin, but remained
febrile. He was admitted to Wuhan Fifth Hospital on Jan 26th 2020 and a throat swab
nucleic acid test was positive for 2019-nCoV. Moxifloxacin 0.4 g and ganciclovir 0.25
g were then given intravenously daily (and continued until Feb 5th 2020). Initially,
his temperature rose to 39°C with a dry cough on Jan 27th,2020 and oxygen saturation
decreased gradually requiring oxygen nasal supplementation. Body temperature dropped
to normal on Jan 29th, however oxygen saturation deteriorated, (75% without supplemental
oxygen after slight activity). CT scan revealed worsening of lung lesions (Figure
1) and oxygen was given through a face mask with improvement of oxygen saturation
to 95%. Intravenous human gamma globulin 10 g / day plus methylprednisolone 80 mg/day
for initiated for 5 consecutive days and other immunosuppressive drugs were held from
Jan 30th to Feb 5th, 2020. After treatment, the patient's symptoms improved, and oxygen
saturation maintained above 96% with nasal cannula oxygen. Intravenous medications
were then stopped, and oral administration of moxifloxacin 0.4 g/day and arbidol (a
non-nucleoside antiviral and immunomodulating drug given for influenza), at a dose
of 0.2 g three times a day was administered for 5 days. Immunosuppressive and antihypertensive
drugs were resumed on Feb 12th, 2020. The patient's temperature normalized for more
than 20 days, without cough for 10 days and preserved oxygen saturation. Two consecutive
RT-PCR throat swabs for 2019-nCoV on Feb 14th and 18th 2020, were negative. CT scan
on Feb 24th showed significant resolution of lung lesions (Figure 1). The patient
was discharged on Feb 27th 2020. Typical imaging demonstrated dynamic progress of
the disease (Figure 1). However, resolution of lung lesions lagged behind symptoms
relief.
Figure 1
Dynamic chest CT manifestations of severe COVID-19 in a heart transplant recipient.
Figure 1
A second heart transplant male recipient aged 43-years old presented to the outpatient
clinic with fever for 2 days on Jan 25th 2020, exhibited mild lung lesions on CT scan,
but a nucleic acid test for 2019-nCoV was positive. The patient was quarantined at
home and then admitted to the hospital on Feb 6th, 2020 following which he was discharged
on Feb 11th when two nucleic acid tests for 2019-nCoV tested negative. (Detailed information
on this patient is in Table 1
).
Table 1
Clinical characteristics of the second patient
Table 1
Date of transplant
May 17th, 2017
Immunosuppression
Tacrolimus 1.5 mg in the morning, 2 mg in the evening mycophenolate mofetil 0.5 g
twice daily
Blood concentration of tacrolimus
8.3 ng/ml
Allograft function
Left ventricular ejection fraction 64%
Comorbidities
Hyperlipidemia and impaired glucose tolerance
Lab test
on Jan 25th2020, WBC 8.2 × 109 /L, Lymphocyte 0.8 × 109 /L, CRP 13.4 mg/Lon Feb 7th2020:
WBC 8.4 × 109 /L, Lymphocyte 1.5 × 109 /L, CRP 1.0 mg/L
RT-PCR of 2019-nCoV (throat swab)
Positive on Jan 28th, negative on Feb 8th and 10th
Treatment
Ceftriaxone sodium 2.0 g and ganciclovir 0.25 g intravenously (Jan 25th to 31st);
Oral moxifloxacin 0.4 g/day and arbidol 0.2 g three times a day (Feb 1st to 10th)
Symptoms evolution
Fever for two days, up to 38.5 degrees CFatigue and poor appetite from Jan 28th to
Feb 5th
Rejection during or after COVID-19
None
Other Complications
None
These cases may represent the first descriptions of COVID-19 in heart transplant recipients
and suggest that presentations appear to be similar to those observed in non-transplant
recipients. We have also followed 200 heart transplant patients in Hubei area by telephone
and found a third confirmed patient who is currently under treatment in another hospital,
but the case details are not available to us and therefore not included in our report.
Whether organ transplant recipients are more susceptible to COVID-19 requires further
large-scale epidemiological investigation, but the presentation pattern and resolution
of the disease using the described supportive measures may serve to inform direction
of care if such patients are encountered elsewhere.
Conflicts of interest and Funding
None.
Related collections
Most cited references1
- Record: found
- Abstract: found
- Article: not found
Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study
Xiaobo Yang, Yuan Yu, Jiqian Xu … (2020)
