- Record: found
- Abstract: found
- Article: found
Alignment between PIN1 Polarity and Microtubule Orientation in the Shoot Apical Meristem Reveals a Tight Coupling between Morphogenesis and Auxin Transport
Read this article at
Abstract
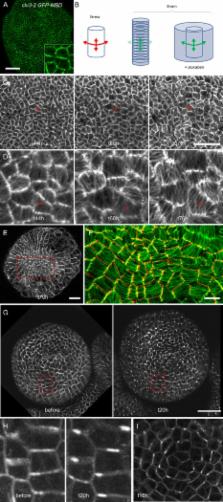
Imaging and computational modeling of the Arabidopsis shoot meristem epidermis suggests that biomechanical signals coordinately regulate auxin efflux carrier distribution and microtubule patterning to orchestrate the extent and directionality of growth.
Abstract
Morphogenesis during multicellular development is regulated by intercellular signaling molecules as well as by the mechanical properties of individual cells. In particular, normal patterns of organogenesis in plants require coordination between growth direction and growth magnitude. How this is achieved remains unclear. Here we show that in Arabidopsis thaliana, auxin patterning and cellular growth are linked through a correlated pattern of auxin efflux carrier localization and cortical microtubule orientation. Our experiments reveal that both PIN1 localization and microtubule array orientation are likely to respond to a shared upstream regulator that appears to be biomechanical in nature. Lastly, through mathematical modeling we show that such a biophysical coupling could mediate the feedback loop between auxin and its transport that underlies plant phyllotaxis.
Author Summary
The proper development of plant organs such as leaves or flowers depends both on localized growth, which can be controlled by the plant hormone auxin, and directional growth, which is dependent on each cell's microtubule cytoskeleton. In this paper we show that at the shoot apex where organs initiate the orientation of the microtubule cytoskeleton is correlated with the orientation of the auxin transporter PIN1, suggesting coordination between growth patterning at the tissue level and directional growth at the cellular level. Recent work has indicated that mechanical signals play a role in orienting the plant microtubule network, and here we show that such signals can also orient PIN1. In addition, we demonstrate through mathematical modeling that an auxin transport system that is coordinated by mechanical signals akin to those we observed in vivo is sufficient to give rise to the patterns of organ outgrowth found in the plant Arabidopsis thaliana.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Improving the photostability of bright monomeric orange and red fluorescent proteins.
- Record: found
- Abstract: found
- Article: not found
Auxin transport inhibitors block PIN1 cycling and vesicle trafficking.
- Record: found
- Abstract: found
- Article: not found