- Record: found
- Abstract: found
- Article: found
The Effect of Osmolytes on Protein Fibrillation
Read this article at
Abstract
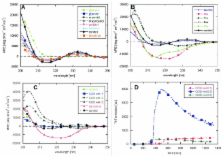
Osmolytes are small molecules that are exploited by cells as a protective system against stress conditions. They favour compact protein states which makes them stabilize globular proteins in vitro and promote folding. Conversely, this preference for compact states promotes aggregation of unstructured proteins. Here we combine a brief review of the effect of osmolytes on protein fibrillation with a report of the effect of osmolytes on the unstructured peptide hormone glucagon. Our results show that osmolytes either accelerate the fibrillation kinetics or leave them unaffected, with the exception of the osmolyte taurine. Furthermore, the osmolytes that affected the shape of the fibrillation time profile led to fibrils with different structure as revealed by CD. The structural changes induced by Pro, Ser and choline- O-sulfate could be due to specific osmolytes binding to the peptides, stabilizing an otherwise labile fibrillation intermediate.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Living with water stress: evolution of osmolyte systems.
- Record: found
- Abstract: found
- Article: not found
Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease.
- Record: found
- Abstract: found
- Article: not found