- Record: found
- Abstract: found
- Article: found
Adeno-associated Virus (AAV) Serotypes Have Distinctive Interactions with Domains of the Cellular AAV Receptor
Read this article at
ABSTRACT
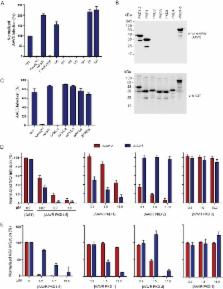
Adeno-associated virus (AAV) entry is determined by its interactions with specific surface glycans and a proteinaceous receptor(s). Adeno-associated virus receptor (AAVR) (also named KIAA0319L) is an essential cellular receptor required for the transduction of vectors derived from multiple AAV serotypes, including the evolutionarily distant serotypes AAV2 and AAV5. Here, we further biochemically characterize the AAV-AAVR interaction and define the domains within the ectodomain of AAVR that facilitate this interaction. By using a virus overlay assay, it was previously shown that the major AAV2 binding protein in membrane preparations of human cells corresponds to a glycoprotein with a molecular mass of 150 kDa. By establishing a purification procedure, performing further protein separation by two-dimensional electrophoresis, and utilizing mass spectrometry, we now show that this glycoprotein is identical to AAVR. While we find that AAVR is an N-linked glycosylated protein, this glycosylation is not a strict requirement for AAV2 binding or functional transduction. Using a combination of genetic complementation with deletion constructs and virus overlay assays with individual domains, we find that AAV2 functionally interacts predominantly with the second Ig-like polycystic kidney disease (PKD) repeat domain (PKD2) present in the ectodomain of AAVR. In contrast, AAV5 interacts primarily through the first, most membrane-distal, PKD domain (PKD1) of AAVR to promote transduction. Furthermore, other AAV serotypes, including AAV1 and -8, require a combination of PKD1 and PKD2 for optimal transduction. These results suggest that despite their shared dependence on AAVR as a critical entry receptor, different AAV serotypes have evolved distinctive interactions with the same receptor.
IMPORTANCE Over the past decade, AAV vectors have emerged as leading gene delivery tools for therapeutic applications and biomedical research. However, fundamental aspects of the AAV life cycle, including how AAV interacts with host cellular factors to facilitate infection, are only partly understood. In particular, AAV receptors contribute significantly to AAV vector transduction efficiency and tropism. The recently identified AAV receptor (AAVR) is a key host receptor for multiple serotypes, including the most studied serotype, AAV2. AAVR binds directly to AAV2 particles and is rate limiting for viral transduction. Defining the AAV-AAVR interface in more detail is important to understand how AAV engages with its cellular receptor and how the receptor facilitates the entry process. Here, we further define AAV-AAVR interactions, genetically and biochemically, and show that different AAV serotypes have discrete interactions with the Ig-like PKD domains of AAVR. These findings reveal an unexpected divergence of AAVR engagement within these parvoviruses.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
High resolution two-dimensional electrophoresis of proteins.
- Record: found
- Abstract: found
- Article: not found
Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy.
- Record: found
- Abstract: found
- Article: not found