- Record: found
- Abstract: found
- Article: found
GHK Peptide Inhibits Bleomycin-Induced Pulmonary Fibrosis in Mice by Suppressing TGFβ1/Smad-Mediated Epithelial-to-Mesenchymal Transition
Read this article at
Abstract
Objective: Idiopathic pulmonary fibrosis is an irreversible and progressive fibrotic lung disease that leads to declines in pulmonary function and, eventually, respiratory failure and has no effective treatment. Gly-His-Lys (GHK) is a tripeptide involved in the processes of tissue regeneration and wound healing and has significant inhibitory effects on transforming growth factor (TGF)-β1 secretion. The effect of GHK on fibrogenesis in pulmonary fibrosis and the exact underlying mechanism have not been studied previously. Thus, this study investigated the effects of GHK on bleomycin (BLM)-induced fibrosis and identified the pathway that is potentially responsible for these effects.
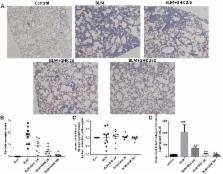
Methods: Intratracheal injections of 3 mg/kg BLM were administered to induce pulmonary fibrosis in C57BL/6 mice. GHK was administered intraperitoneally at doses of 2.6, 26, and 260 μg/ml/day every other day from the 4th to the 21st day after BLM instillation. Three weeks after BLM instillation, pulmonary injury and pulmonary fibrosis was evaluated by the hematoxylin-eosin (HE) and Masson’s trichrome (MT) staining. Chronic inflammation index was used for the histological assessments by two pathologists blindly to each other. Tumor necrosis factor (TNF)-α and IL-6 levels in BALF and myeloperoxidase (MPO) activity in lung extracts were measured. For the pulmonary fibrosis evaluation, the fibrosis index calculated based on MT staining, collagen deposition and active TGF-β1 expression detected by ELISA, and the expression of TGF-β1, α-smooth muscle actin (SMA), fibronectin, MMP-9, and TIMP-1 by western blotting. The epithelial mesenchymal transition index, E-cadherin, and vimentin was also detected by western blot. The statistical analysis was performed by one-way ANOVA and the comparison between different groups were performed.
Results: Treatment with GHK at all three doses reduced inflammatory cell infiltration and interstitial thickness and attenuated BLM-induced pulmonary fibrosis in mice. GHK treatment significantly improved collagen deposition, and MMP-9/TIMP-1 imbalances in lung tissue and also reduced TNF-α, IL-6 expression in bronchoalveolar lavage fluid (BALF) and MPO in lung extracts. Furthermore, GHK reversed BLM-induced increases in TGF-β1, p-Smad2, p-Smad-3 and insulin-like growth factor-1 (IGF-1) expression.
Conclusion: GHK inhibits BLM-induced fibrosis progression, the inflammatory response and EMT via the TGF-β1/Smad 2/3 and IGF-1 pathway. Thus, GHK may be a potential treatment for pulmonary fibrosis.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease.

- Record: found
- Abstract: found
- Article: found
Mechanisms of TGFβ-Induced Epithelial–Mesenchymal Transition
- Record: found
- Abstract: found
- Article: not found