- Record: found
- Abstract: found
- Article: not found
Effects of shakuyakukanzoto and its absorbed components on twitch contractions induced by physiological Ca 2+ release in rat skeletal muscle
Read this article at
Abstract
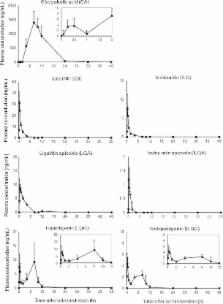
Shakuyakukanzoto (SKT) is a kampo medicine composed of equal proportions of Glycyrrhizae radix ( G. radix) and Paeoniae radix ( P. radix). A double-blind study reported that SKT significantly ameliorated painful muscle cramp in cirrhosis patients without the typical severe side effects of muscle weakness and central nervous system (CNS) depression. Previous basic studies reported that SKT and its active components induced relaxation by a direct action on skeletal muscle and that SKT did not depress CNS functions; however, why SKT has a lower incidence of muscle weakness remains unknown. In the present study, we investigated which components are absorbed into the blood of rats after a single oral administration of SKT to identify the active components of SKT. We also investigated the effects of SKT and its components on the twitch contraction induced by physiological Ca 2+ release. Our study demonstrated that SKT and five G. radix isolates, which are responsible for the antispasmodic effect of SKT, did not inhibit the twitch contraction in contrast to dantrolene sodium, a direct-acting peripheral muscle relaxant, indicating that the mechanisms of muscle contraction of SKT and dantrolene in skeletal muscle differ. These findings suggest that SKT does not reduce the contractile force in skeletal muscle under physiological conditions, i.e., SKT may have a low risk of causing muscle weakness in clinical use. Considering that most muscle relaxants and anticonvulsants cause various harmful side effects such as weakness and CNS depression, SKT appears to have a benign safety profile.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin.
- Record: found
- Abstract: found
- Article: not found
Dantrolene--a review of its pharmacology, therapeutic use and new developments.
- Record: found
- Abstract: found
- Article: not found
