- Record: found
- Abstract: found
- Article: found
A New Immortalized Human Alveolar Epithelial Cell Model to Study Lung Injury and Toxicity on a Breathing Lung-On-Chip System
Read this article at
Abstract
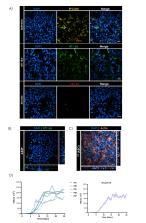
The evaluation of inhalation toxicity, drug safety and efficacy assessment, as well as the investigation of complex disease pathomechanisms, are increasingly relying on in vitro lung models. This is due to the progressive shift towards human-based systems for more predictive and translational research. While several cellular models are currently available for the upper airways, modelling the distal alveolar region poses several constraints that make the standardization of reliable alveolar in vitro models relatively difficult. In this work, we present a new and reproducible alveolar in vitro model, that combines a human derived immortalized alveolar epithelial cell line ( AXiAEC) and organ-on-chip technology mimicking the lung alveolar biophysical environment ( AXlung-on-chip). The latter mimics key features of the in vivo alveolar milieu: breathing-like 3D cyclic stretch (10% linear strain, 0.2 Hz frequency) and an ultrathin, porous and elastic membrane. AXiAECs cultured on-chip were characterized for their alveolar epithelial cell markers by gene and protein expression. Cell barrier properties were examined by TER (Transbarrier Electrical Resistance) measurement and tight junction formation. To establish a physiological model for the distal lung, AXiAECs were cultured for long-term at air-liquid interface (ALI) on-chip. To this end, different stages of alveolar damage including inflammation (via exposure to bacterial lipopolysaccharide) and the response to a profibrotic mediator (via exposure to Transforming growth factor β1) were analyzed. In addition, the expression of relevant host cell factors involved in SARS-CoV-2 infection was investigated to evaluate its potential application for COVID-19 studies. This study shows that AXiAECs cultured on the AXlung-on-chip exhibit an enhanced in vivo-like alveolar character which is reflected into: 1) Alveolar type 1 (AT1) and 2 (AT2) cell specific phenotypes, 2) tight barrier formation (with TER above 1,000 Ω cm 2) and 3) reproducible long-term preservation of alveolar characteristics in nearly physiological conditions (co-culture, breathing, ALI). To the best of our knowledge, this is the first time that a primary derived alveolar epithelial cell line on-chip representing both AT1 and AT2 characteristics is reported. This distal lung model thereby represents a valuable in vitro tool to study inhalation toxicity, test safety and efficacy of drug compounds and characterization of xenobiotics.
Related collections
Most cited references114
- Record: found
- Abstract: found
- Article: not found
SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor
- Record: found
- Abstract: found
- Article: not found
The basics of epithelial-mesenchymal transition.
- Record: found
- Abstract: found
- Article: not found
