- Record: found
- Abstract: found
- Article: found
Exploring the Chemodiversity and Biological Activities of the Secondary Metabolites from the Marine Fungus Neosartorya pseudofischeri
Read this article at
Abstract
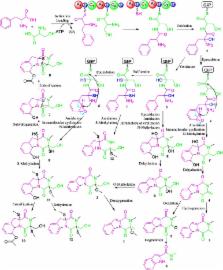
The production of fungal metabolites can be remarkably influenced by various cultivation parameters. To explore the biosynthetic potentials of the marine fungus, Neosartorya pseudofischeri, which was isolated from the inner tissue of starfish Acanthaster planci, glycerol-peptone-yeast extract (GlyPY) and glucose-peptone-yeast extract (GluPY) media were used to culture this fungus. When cultured in GlyPY medium, this fungus produced two novel diketopiperazines, neosartins A and B ( 1 and 2), together with six biogenetically-related known diketopiperazines,1,2,3,4-tetrahydro-2,3-dimethyl-1,4-dioxopyrazino[1,2-a]indole ( 3), 1,2,3,4-tetrahydro-2-methyl-3-methylene-1,4-dioxopyrazino[1,2-a]indole ( 4), 1,2,3,4-tetrahydro-2-methyl-1,3,4-trioxopyrazino[1,2-a] indole ( 5), 6-acetylbis(methylthio)gliotoxin ( 10), bisdethiobis(methylthio)gliotoxin ( 11), didehydrobisdethiobis(methylthio)gliotoxin ( 12) and N-methyl-1 H-indole-2-carboxamide ( 6). However, a novel tetracyclic-fused alkaloid, neosartin C ( 14), a meroterpenoid, pyripyropene A ( 15), gliotoxin ( 7) and five known gliotoxin analogues, acetylgliotoxin ( 8), reduced gliotoxin ( 9), 6-acetylbis(methylthio)gliotoxin ( 10), bisdethiobis(methylthio) gliotoxin ( 11) and bis- N-norgliovictin ( 13), were obtained when grown in glucose-containing medium (GluPY medium). This is the first report of compounds 3, 4, 6, 9, 10 and 12 as naturally occurring. Their structures were determined mainly by MS, 1D and 2D NMR data. The possible biosynthetic pathways of gliotoxin-related analogues and neosartin C were proposed. The antibacterial activity of compounds 2– 14 and the cytotoxic activity of compounds 4, 5 and 7– 13 were evaluated. Their structure-activity relationships are also preliminarily discussed.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Transannular disulfide formation in gliotoxin biosynthesis and its role in self-resistance of the human pathogen Aspergillus fumigatus.

- Record: found
- Abstract: found
- Article: found
Epigenetic Tailoring for the Production of Anti-Infective Cytosporones from the Marine Fungus Leucostoma persoonii
- Record: found
- Abstract: found
- Article: not found