- Record: found
- Abstract: found
- Article: found
A Tetraspecific VHH-Based Neutralizing Antibody Modifies Disease Outcome in Three Animal Models of Clostridium difficile Infection
Read this article at
Abstract
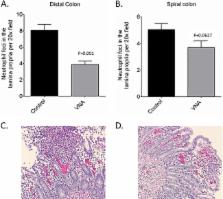
Clostridium difficile infection (CDI), a leading cause of nosocomial infection, is a serious disease in North America, Europe, and Asia. CDI varies greatly from asymptomatic carriage to life-threatening diarrhea, toxic megacolon, and toxemia. The incidence of community-acquired infection has increased due to the emergence of hypervirulent antibiotic-resistant strains. These new strains contribute to the frequent occurrence of disease relapse, complicating treatment, increasing hospital stays, and increasing morbidity and mortality among patients. Therefore, it is critical to develop new therapeutic approaches that bypass the development of antimicrobial resistance and avoid disruption of gut microflora. Here, we describe the construction of a single heteromultimeric VHH-based neutralizing agent (VNA) that targets the two primary virulence factors of Clostridium difficile, toxins A (TcdA) and B (TcdB). Designated VNA2-Tcd, this agent has subnanomolar toxin neutralization potencies for both C. difficile toxins in cell assays. When given systemically by parenteral administration, VNA2-Tcd protected against CDI in gnotobiotic piglets and mice and to a lesser extent in hamsters. Protection from CDI was also observed in gnotobiotic piglets treated by gene therapy with an adenovirus that promoted the expression of VNA2-Tcd.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
The changing epidemiology of Clostridium difficile infections.
- Record: found
- Abstract: found
- Article: not found
Selection and identification of single domain antibody fragments from camel heavy-chain antibodies.
- Record: found
- Abstract: found
- Article: not found
