- Record: found
- Abstract: found
- Article: found
Quantitative properties and receptor reserve of the DAG and PKC branch of G q-coupled receptor signaling
Read this article at
Abstract
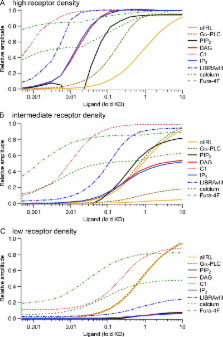
G q protein–coupled receptors (G qPCRs) of the plasma membrane activate the phospholipase C (PLC) signaling cascade. PLC cleaves the membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP 2) into the second messengers diacylgycerol (DAG) and inositol 1,4,5-trisphosphate (IP 3), leading to calcium release, protein kinase C (PKC) activation, and in some cases, PIP 2 depletion. We determine the kinetics of each of these downstream endpoints and also ask which is responsible for the inhibition of KCNQ2/3 (K V7.2/7.3) potassium channels in single living tsA-201 cells. We measure DAG production and PKC activity by Förster resonance energy transfer–based sensors, and PIP 2 by KCNQ2/3 channels. Fully activating endogenous purinergic receptors by uridine 5′triphosphate (UTP) leads to calcium release, DAG production, and PKC activation, but no net PIP 2 depletion. Fully activating high-density transfected muscarinic receptors (M 1Rs) by oxotremorine-M (Oxo-M) leads to similar calcium, DAG, and PKC signals, but PIP 2 is depleted. KCNQ2/3 channels are inhibited by the Oxo-M treatment (85%) and not by UTP (<1%), indicating that depletion of PIP 2 is required to inhibit KCNQ2/3 in response to receptor activation. Overexpression of A kinase–anchoring protein (AKAP)79 or calmodulin (CaM) does not increase KCNQ2/3 inhibition by UTP. From these results and measurements of IP 3 and calcium presented in our companion paper (Dickson et al. 2013. J. Gen. Physiol. http://dx.doi.org/10.1085/jgp.201210886), we extend our kinetic model for signaling from M 1Rs to DAG/PKC and IP 3/calcium signaling. We conclude that calcium/CaM and PKC-mediated phosphorylation do not underlie dynamic KCNQ2/3 channel inhibition during G qPCR activation in tsA-201 cells. Finally, our experimental data provide indirect evidence for cleavage of PI(4)P by PLC in living cells, and our modeling revisits/explains the concept of receptor reserve with measurements from all steps of G qPCR signaling.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Inositol trisphosphate receptor Ca2+ release channels.
- Record: found
- Abstract: found
- Article: not found
Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels.
- Record: found
- Abstract: found
- Article: not found