- Record: found
- Abstract: found
- Article: found
All-Trans Retinoic Acid Modulates TLR4/NF- κB Signaling Pathway Targeting TNF- α and Nitric Oxide Synthase 2 Expression in Colonic Mucosa during Ulcerative Colitis and Colitis Associated Cancer
Read this article at
Abstract
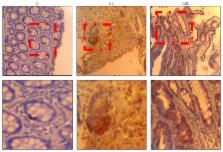
Colitis associated cancer (CAC) is the colorectal cancer (CRC) subtype that is associated with bowel disease such as ulcerative colitis (UC). The data on role of NF- κB signaling in development and progression of CAC were derived from preclinical studies, whereas data from human are rare. The aim of this work was to study the contribution of NF- κB pathway during UC and CAC, as well as the immunomodulatory effect of all-trans retinoic acid (AtRA). We analyzed the expression of NOS2, TNF- α, TLR4, and NF- κB, in colonic mucosa. We also studied NO/TNF- α modulation by LPS in colonic mucosa pretreated with AtRA. A marked increase in TLR4, NF- κB, TNF- α, and NOS2 expression was reported in colonic mucosa. The relationship between LPS/TLR4 and TNF- α/NO production, as well as the role of NF- κB signaling, was confirmed by ex vivo experiments and the role of LPS/TLR4 in NOS2/TNF- α induction through NF- κB pathway was suggested. AtRA downregulates NOS2 and TNF- α expression. Collectively, our study indicates that AtRA modulates in situ LPS/TLR4/NF- κB signaling pathway targeting NOS2 and TNF- α expression. Therefore, we suggest that AtRA has a potential value in new strategies to improve the current therapy, as well as in the clinical prevention of CAC development and progression.
Related collections
Most cited references76
- Record: found
- Abstract: found
- Article: not found
LPS-TLR4 Signaling to IRF-3/7 and NF-κB Involves the Toll Adapters TRAM and TRIF
- Record: found
- Abstract: found
- Article: not found