- Record: found
- Abstract: found
- Article: found
Pharmacokinetic evaluation of Chalcone derivatives with antimalarial activity in New Zealand White Rabbits
Read this article at
Abstract
Objective
Malaria is a major global health concern with the urgent need for new treatment alternatives due to the alarming increase of drug-resistant Plasmodium strains. Chalcones and its derivatives are important pharmacophores showing antimalarial activity. Determination of the pharmacokinetic variables at the preliminary step of drug development for any drug candidates is an essential component of in vivo antimalarial efficacy tests. Substandard pharmacokinetic variables are often responsible for insufficient therapeutic effect. Therefore, three chalcone derivatives, 1, 2, and 3, having antimalarial potency were studied further for potential therapeutic efficacy.
Results
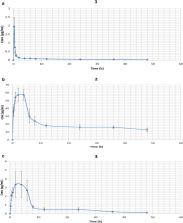
In vivo pharmacokinetic studies of these three derivatives were performed on New Zealand White rabbits. The three derivatives were administered intra-peritoneally or orally at effective dose concentration and blood samples at different time points were collected. The determination of drug concentration was done through reverse phase-high performance liquid chromatography. The peak plasma concentration of derivative 1, 2, and 3 were 1.96 ± 0.46 µg/mL (intraperitoneal route), 69.89 ± 5.49 µg/mL (oral route), and 3.74 ± 1.64 µg/mL (oral route). The results indicate a very low bioavailability of these derivatives. The present study gives a benchmark to advance the investigation of more derivatives in order to revamp the pharmacokinetic variables while maintaining both potency and metabolic constancy.
Related collections
Most cited references26
- Record: found
- Abstract: not found
- Article: not found
Exploring Pharmacological Significance of Chalcone Scaffold: A Review
- Record: found
- Abstract: found
- Article: not found
Artemisinin-Resistant Plasmodium falciparum Malaria.
- Record: found
- Abstract: found
- Article: not found
