- Record: found
- Abstract: found
- Article: found
Bothrops bilineatus: An Arboreal Pitviper in the Amazon and Atlantic Forest
Read this article at
Abstract
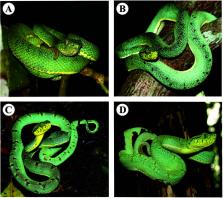
The two-striped forest-pitviper ( Bothrops bilineatus) is an arboreal snake that is currently represented by two subspecies ( B. b. bilineatus and B. b. smaragdinus) that comprise a species complex, and its distribution is in the Amazon and the Atlantic Forest. The rarity of encounters with this snake is reflected in the low occurrence of cases of snakebites throughout its geographic distribution and the resulting low number of published clinical reports. However, in some areas, B. bilineatus proves to be more frequent and causes envenomations in a greater proportion. Herein, we review the main aspects of the species complex B. bilineatus, including its biology, ecology, taxonomy, morphology, genetic and molecular studies, geographic distribution, conservation status, venom, pathophysiology and clinical aspects, and epidemiology. In addition, the different antivenoms available for the treatment of envenomations caused by B. bilineatus are presented along with suggestions for future studies that are needed for a better understanding of the snakebites caused by this snake.
Related collections
Most cited references137
- Record: found
- Abstract: found
- Article: not found
The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms.
- Record: found
- Abstract: found
- Article: not found
Insights into and speculations about snake venom metalloproteinase (SVMP) synthesis, folding and disulfide bond formation and their contribution to venom complexity.
- Record: found
- Abstract: found
- Article: not found