- Record: found
- Abstract: found
- Article: found
Ultrasound‐Stimulated “Exocytosis” by Cell‐Like Microbubbles Enhances Antibacterial Species Penetration and Immune Activation Against Implant Infection

Read this article at
Abstract
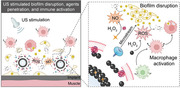
Host immune systems serving as crucial defense lines are vital resisting mechanisms against biofilm‐associated implant infections. Nevertheless, biofilms hinder the penetration of anti‐bacterial species, inhibit phagocytosis of immune cells, and frustrate host inflammatory responses, ultimately resulting in the weakness of the host immune system for biofilm elimination. Herein, a cell‐like construct is developed through encapsulation of erythrocyte membrane fragments on the surface of Fe 3O 4 nanoparticle‐fabricated microbubbles and then loaded with hydroxyurea (EMB‐Hu). Under ultrasound (US) stimulation, EMB‐Hu undergoes a stable oscillation manner to act in an “exocytosis” mechanism for disrupting biofilm, releasing agents, and enhancing penetration of catalytically generated anti‐bacterial species within biofilms. Additionally, the US‐stimulated “exocytosis” by EMB‐Hu can activate pro‐inflammatory macrophage polarization and enhance macrophage phagocytosis for clearance of disrupted biofilms. Collectively, this work has exhibited cell‐like microbubbles with US‐stimulated “exocytosis” mechanisms to overcome the biofilm barrier and signal macrophages for inflammatory activation, finally achieving favorable therapeutic effects against implant infections caused by methicillin‐resistant Staphylococcus aureus (MRSA) biofilms.
Abstract
Under ultrasound (US) stimulation, erythrocyte membrane fragments encapsulated microbubbles loaded with hydroxyurea (EMB‐Hu) can contract in an oscillatory manner for agent release. Such US‐stimulated “exocytosis” by EMB‐Hu can disrupt biofilm structure by the generation of microstream, enhance the penetration of antibacterial species within biofilms, and also activate macrophages for efficient elimination of residual biofilms during implant infection.