- Record: found
- Abstract: found
- Article: found
lncRNA LINC01296 regulates the proliferation, metastasis and cell cycle of osteosarcoma through cyclin D1
Read this article at
Abstract
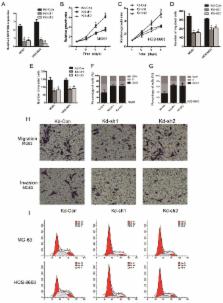
Accumulating evidence has indicated that aberrant expression of long non-coding RNAs (lncRNAs) is an important oncogenic factor. The aim of the present study was to investigate the role of LINC01296, an lncRNA that exerts a tumor-promoting function in many cancers, in the regulation of proliferation, metastasis and the cell cycle of osteosarcoma. The expression of LINC01296 in osteosarcoma tissues and adjacent healthy tissues of 30 patients was analyzed by quantitative real-time PCR (qRT-PCR). The relationship between LINC01296 expression and the survival of patients with osteosarcoma was also explored. The expression levels of LINC01296 in osteosarcoma cells and normal cells were compared. LINC01296 knockdown and overexpression were performed in MG63 and HOS8603 osteosarcoma cells by transfecting LINC01296 shRNA and an expression plasmid respectively, followed by investigation of the changes on cell proliferation, migration, apoptosis and cell cycle arrest. Western blotting was used to analyze the changes of cell cycle regulators. Cyclin D1 knockdown and overexpression were carried out to verify the interaction between LINC01296 and cyclin D1. LINC01296 overexpression was demonstrated as a biomarker of osteosarcoma, which was closely correlated with the poor survival of patients with osteosarcoma. A high expression of LINC01296 was observed in osteosarcoma cells, which was closely associated with enhanced proliferation, invasion, and migration of osteosarcoma cells. Cyclin D1 expression was positively correlated with the expression of LINC01296 in osteosarcoma cells. Cyclin D1 knockdown or overexpression played a deterministic role in mediating the effect of LINC01296 on osteosarcoma cells. LINC01296 is an oncogenic lncRNA in osteosarcoma. The proliferation, invasion and migration of osteosarcoma cells could be effectively retarded by inhibition of LINC01296. The cancer-promoting effect of LINC01296 on osteosarcoma was determined by cyclin D1.
Related collections
Most cited references16
- Record: found
- Abstract: found
- Article: not found
Cyclin D1, cancer progression, and opportunities in cancer treatment.

- Record: found
- Abstract: found
- Article: found
Identification of the long non-coding RNA H19 in plasma as a novel biomarker for diagnosis of gastric cancer

- Record: found
- Abstract: found
- Article: found