- Record: found
- Abstract: found
- Article: found
Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR Inhibitors: Rationale and Importance to Inhibiting These Pathways in Human Health
Read this article at
Abstract
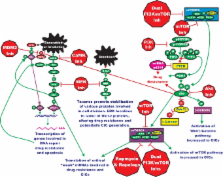
The Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascades are often activated by genetic alterations in upstream signaling molecules such as receptor tyrosine kinases (RTK). Integral components of these pathways, Ras, B-Raf, PI3K, and PTEN are also activated/inactivated by mutations. These pathways have profound effects on proliferative, apoptotic and differentiation pathways. Dysregulation of these pathways can contribute to chemotherapeutic drug resistance, proliferation of cancer initiating cells (CICs) and premature aging. This review will evaluate more recently described potential uses of MEK, PI3K, Akt and mTOR inhibitors in the proliferation of malignant cells, suppression of CICs, cellular senescence and prevention of aging. Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt/mTOR pathways play key roles in the regulation of normal and malignant cell growth. Inhibitors targeting these pathways have many potential uses from suppression of cancer, proliferative diseases as well as aging.
Related collections
Most cited references254
- Record: found
- Abstract: found
- Article: not found
Specificity and mechanism of action of some commonly used protein kinase inhibitors.
- Record: found
- Abstract: found
- Article: not found
Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy.
- Record: found
- Abstract: found
- Article: not found