- Record: found
- Abstract: found
- Article: found
Arresting vertical transmission of hepatitis B virus (AVERT-HBV) in pregnant women and their neonates in the Democratic Republic of the Congo: a feasibility study
Read this article at
Summary
Background
Hepatitis B virus (HBV) remains endemic throughout sub-Saharan Africa despite the widespread availability of effective childhood vaccines. In the Democratic Republic of the Congo, HBV treatment and birth-dose vaccination programmes are not established. We, therefore, aimed to evaluate the feasibility and acceptability of adding HBV testing and treatment of pregnant women as well as the birth-dose vaccination of HBV-exposed infants to the HIV prevention of mother-to-child transmission programme infrastructure in the Democratic Republic of the Congo.
Methods
We did a feasibility study in two maternity centres in Kinshasa: Binza and Kingasani. Using the already established HIV prevention of mother-to-child transmission programme at these two maternity centres, we screened pregnant women for HBV infection at routine prenatal care registration. Those who tested positive and had a gestational age of 24 weeks or less were included in this study. Eligible pregnant women with a high viral load (≥200 000 IU/mL or HBeAg positivity, or both) were considered as having HBV of high risk of mother-to-child transmission and initiated on oral tenofovir disoproxil fumarate (300 mg/day) between 28 weeks and 32 weeks of gestation and continued through 12 weeks post partum. All HBV-exposed infants received a birth-dose of monovalent HBV vaccine (Euvax-B Pediatric: Sanofi Pasteur, Seoul, South Korea; 0·5 mL) within 24 h of life. All women were followed up for 24 weeks post partum, when they completed an exit questionnaire that assessed the acceptability of study procedures. The primary outcomes were the feasibility of screening pregnant women to identify those at high risk for HBV mother-to-child transmission and to provide them with antiviral prophylaxis, the feasibility of administrating the birth-dose vaccine to exposed infants, and the acceptability of this prevention programme. This study is registered with ClinicalTrials.gov, NCT03567382.
Findings
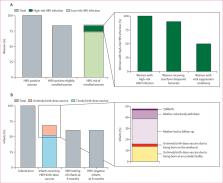
Between Sept 24, 2018, and Feb 22, 2019, 4016 women were approached and screened. Of these pregnant women, 109 (2∙7%) were positive for HBsAg. Of the 109 women, 91 (83%) met the eligibility criteria for participation. However, only data from 90 women—excluding one woman who had a false pregnancy—were included in the study analysis. The median overall age of the enrolled women was 31 years (IQR 25–34) and the median overall gestational age was 19 weeks (15–22). Ten (11%) of 91 women evaluated had high-risk HBV infection. Nine (90%) of the ten pregnant women with high-risk HBV infection received tenofovir disoproxil fumarate and one (10%) refused therapy and withdrew from the study; five (56%) of the nine women achieved viral suppression (ie, <200 000 IU/mL) on tenofovir disoproxil fumarate therapy by the time of delivery and the remaining four (44%) had decreased viral loads from enrolment to delivery. A total of 88 infants were born to the 90 enrolled women. Of the 88 infants, 60 (68%) received a birth-dose vaccine; of these, 46 (77%) received a timely birth-dose vaccine. No cases of HBV mother-to-child transmission were observed. No serious adverse events associated with tenofovir disoproxil fumarate nor with the birth-dose vaccine were reported. Only one (11%) of nine women reported dizziness during the course of tenofovir disoproxil fumarate therapy. The study procedures were considered highly acceptable (>80%) among mothers.
Interpretation
Adding HBV screening and treatment of pregnant women and infant birth-dose vaccination to existing HIV prevention of mother-to-child transmission platforms is feasible in countries such as the Democratic Republic of the Congo. Birth-dose vaccination against HBV infection integrated within the current Expanded Programme on Immunisation and HIV prevention of mother-to-child transmission programme could accelerate progress toward HBV elimination in Africa.
Related collections
Most cited references28
- Record: found
- Abstract: not found
- Article: not found
AASLD guidelines for treatment of chronic hepatitis B.
- Record: found
- Abstract: not found
- Article: not found
EASL clinical practice guidelines: Management of chronic hepatitis B virus infection.
- Record: found
- Abstract: found
- Article: not found